Visualization of an Accelerated Electrochemical Reaction under an Enhanced Electric Field
- PMID: 33681811
- PMCID: PMC7907821
- DOI: 10.34133/2021/1742919
Visualization of an Accelerated Electrochemical Reaction under an Enhanced Electric Field
Abstract
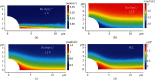
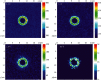
Locally enhanced electric fields produced by high-curvature structures have been reported to boost the charge transport process and improve the relevant catalytic activity. However, no visual evidence has been achieved to support this new electrochemical mechanism. Here, accelerated electrochemiluminescence (ECL) reactions emitting light are visualized for the first time at the heterogeneous interfaces between microbowls and the supporting electrode surface. The simulation result shows that the electric intensity at the interface with a high curvature is 40-fold higher than that at the planar surface. Consequently, local high electric fields concentrate reactive species to the heterogeneous interfaces and efficiently promote the charge transport reactions, which directly leads to the enhancement of ECL emission surrounding the microbowls. Additionally, the potential to induce visual ECL from a ruthenium complex drops to 0.9 V, which further illustrates the promotion of an electrochemical reaction with the aid of an enhanced electric field. This important visualization of electric field boosted electrochemical reactions helps to establish the proposed electron transfer mechanism and provide an alternative strategy to improve electrocatalytic efficiency.
Copyright © 2021 Chen Cui et al.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Ji D., Sun J., Tian L., et al. Engineering of the heterointerface of porous carbon nanofiber–supported nickel and manganese oxide nanoparticle for highly efficient bifunctional oxygen catalysis. Advanced Functional Materials. 2020;30(13, article 1910568) doi: 10.1002/adfm.201910568. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

