Healing rates after rotator cuff repair for patients taking either celecoxib or placebo: a double-blind randomized controlled trial
- PMID: 33681844
- PMCID: PMC7910746
- DOI: 10.1016/j.jseint.2020.10.011
Healing rates after rotator cuff repair for patients taking either celecoxib or placebo: a double-blind randomized controlled trial
Abstract
Background: Use of anti-inflammatory medications (NSAIDs) is an important component of multimodal pain control after orthopedic procedures to avoid opioid overutilization and abuse. However, the deleterious effects of NSAIDs on tendon healing are of particular concern in rotator cuff repair (RCR). The purpose of this study was to evaluate the effect of celecoxib or placebo on healing rates after RCR when administered in the perioperative and immediate postoperative period using MRI evaluation at one year postoperatively. A secondary aim was to determine whether clinical differences existed between patients with intact or non-intact repairs.
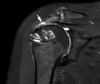
Methods: Patients aged ≤65 years with partial- or full-thickness rotator cuff tear (<25x25 mm) were randomized to receive celecoxib 400 mg or placebo 1 hour before the procedure and 200mg bid for 3 weeks postoperatively. All patients were treated as clinically indicated at the time of surgery and followed standard postoperative protocol. Repair integrity was evaluated with MRI using the Sugaya classification for repair integrity. Data were analyzed using multivariable logistic regression by intent to treat.
Results: Seventy-nine patients were enrolled; 21 were lost to follow-up, 6 did not have cuff repair, 5 were revised, and 2 declined follow-up, leaving 45 patients with one-year follow-up. Five of these patients did not complete MRI, leaving 40 patients for review. Eighteen of 20 patients (90%) who received celecoxib completed all doses of study medication as did 15 of 20 patients (75%) who received placebo. The patient groups were similar for demographics, clinical results, and healing rate. After adjusting for tear size, no statistically significant difference in healing rate was found between groups, with 10 of 20 celecoxib patients (50%) having intact repair at 1 year compared with 14 of 20 placebo patients (70%) (OR = 0.53, 95% CI: 0.14, 2.08, P = 0.35).
Conclusion: Half of the patients who received celecoxib had an intact repair compared with 70% intact repair for patients receiving placebo. Although not statistically significant in this small study, larger studies are needed to clarify this important clinical concern. The authors do not recommend use of celecoxib for postoperative pain control after RCR.
Keywords: Rotator cuff repair; arthroscopic rotator cuff repair; celecoxib; healing rate; pain management; rotator cuff tear.
© 2020 The Authors.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
