Safety and effectiveness of mycophenolate mofetil associated with tacrolimus for liver transplantation immunosuppression: a systematic review and meta-analysis of randomized controlled trials
- PMID: 33681947
- PMCID: PMC7920399
- DOI: 10.6061/clinics/2021/e2597
Safety and effectiveness of mycophenolate mofetil associated with tacrolimus for liver transplantation immunosuppression: a systematic review and meta-analysis of randomized controlled trials
Abstract
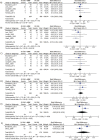
A combination of immunosuppressants may improve outcomes due to the synergistic effect of their different action mechanisms. Currently, there is no consensus regarding the best immunosuppressive protocol after liver transplantation. This review aimed to evaluate the effectiveness and safety of tacrolimus associated with mycophenolate mofetil (MMF) in patients undergoing liver transplantation. We performed a systematic review and meta-analysis of randomized clinical trials. Eight randomized trials were included. The proportion of patients with at least one adverse event related to the immunosuppression scheme with tacrolimus associated with MMF was 39.9%. The tacrolimus with MMF immunosuppression regimen was superior in preventing acute cellular rejection compared with that of tacrolimus alone (risk difference [RD]=-0.11; p =0.001). The tacrolimus plus MMF regimen showed no difference in the risk of adverse events compared to that of tacrolimus alone (RD=0.7; p=0.66) and cyclosporine plus MMF (RD=-0.7; p=0.37). Patients undergoing liver transplantation who received tacrolimus plus MMF had similar adverse events when compared to patients receiving other evaluated immunosuppressive regimens and had a lower risk of acute rejection than those receiving in the monodrug tacrolimus regimen.
Conflict of interest statement
No potential conflict of interest was reported.
Figures






Similar articles
-
Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation.Transplantation. 2000 Mar 15;69(5):834-41. doi: 10.1097/00007890-200003150-00028. Transplantation. 2000. PMID: 10755536 Clinical Trial.
-
New strategies using 'low-dose' mycophenolate mofetil to reduce acute rejection in patients following kidney transplantation.J Transpl Coord. 1999 Jun;9(2):114-8. doi: 10.7182/prtr.1.9.2.t4l566l63m0g1126. J Transpl Coord. 1999. PMID: 10703393 Clinical Trial.
-
Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months.Transplantation. 2003 Apr 27;75(8):1213-20. doi: 10.1097/01.TP.0000062837.99400.60. Transplantation. 2003. PMID: 12717205 Clinical Trial.
-
Efficacy of immunosuppression monotherapy after liver transplantation: a meta-analysis.World J Gastroenterol. 2014 Sep 14;20(34):12330-40. doi: 10.3748/wjg.v20.i34.12330. World J Gastroenterol. 2014. PMID: 25232269 Free PMC article. Review.
-
Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option?Paediatr Drugs. 2011 Feb 1;13(1):49-69. doi: 10.2165/11538530-000000000-00000. Paediatr Drugs. 2011. PMID: 21162600 Review.
Cited by
-
Paradox inflammatory reaction such as appendicitis epiploica and diverticulitis of the sigmoid colon under ongoing immunosuppression after previous liver transplantation (LTx).Innov Surg Sci. 2023 Sep 15;8(2):123-128. doi: 10.1515/iss-2023-0038. eCollection 2023 Jun. Innov Surg Sci. 2023. PMID: 38058776 Free PMC article.
-
Impact of COVID-19 Infection on Liver Transplant Recipients: Does It Make Any Difference?Cureus. 2022 Feb 28;14(2):e22687. doi: 10.7759/cureus.22687. eCollection 2022 Feb. Cureus. 2022. PMID: 35386162 Free PMC article.
-
Regulation of the immune microenvironment and immunotherapy after liver transplantation.Front Immunol. 2025 May 12;16:1602877. doi: 10.3389/fimmu.2025.1602877. eCollection 2025. Front Immunol. 2025. PMID: 40421010 Free PMC article. Review.
-
Multiple Shades of Gray-Macrophages in Acute Allograft Rejection.Int J Mol Sci. 2023 May 4;24(9):8257. doi: 10.3390/ijms24098257. Int J Mol Sci. 2023. PMID: 37175964 Free PMC article. Review.
-
From bench to bed: deep insights the tacrolimus-induced diabetes and gut microbiota dysbiosis.Eur J Med Res. 2025 Aug 11;30(1):731. doi: 10.1186/s40001-025-03000-9. Eur J Med Res. 2025. PMID: 40790600 Free PMC article. Review.
References
-
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Marsh JW, McCauley J, et al. A prospective, randomized trial of tacrolimus/prednisone versus tacrolimus/prednisone/mycophenolate mofetil in renal transplant recipients. Transplantation. 1999;67(3):411–5. doi: 10.1097/00007890-199902150-00012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

