Delayed Adaptive Radiation among New Zealand Stream Fishes: Joint Estimation of Divergence Time and Trait Evolution in a Newly Delineated Island Species Flock
- PMID: 33682001
- PMCID: PMC8677556
- DOI: 10.1093/sysbio/syab014
Delayed Adaptive Radiation among New Zealand Stream Fishes: Joint Estimation of Divergence Time and Trait Evolution in a Newly Delineated Island Species Flock
Erratum in
-
Corrigendum to: Delayed Adaptive Radiation among New Zealand Stream Fishes: Joint Estimation of Divergence Time and Trait Evolution in a Newly Delineated Island Species Flock.Syst Biol. 2022 Apr 19;71(3):775. doi: 10.1093/sysbio/syac013. Syst Biol. 2022. PMID: 35255497 Free PMC article. No abstract available.
Abstract
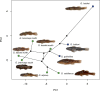
Adaptive radiations are generally thought to occur soon after a lineage invades a region offering high levels of ecological opportunity. However, few adaptive radiations beyond a handful of exceptional examples are known, so a comprehensive understanding of their dynamics is still lacking. Here, we present a novel case of an island species flock of freshwater fishes with a radically different tempo of adaptive history than that found in many popular evolutionary model systems. Using a phylogenomic data set combined with simultaneous Bayesian estimation of divergence times and trait-based speciation and extinction models, we show that the New Zealand Gobiomorphus gudgeons comprise a monophyletic assemblage, but surprisingly, the radiation did not fully occupy freshwater habitats and explosively speciate until more than 10 myr after the lineage invaded the islands. This shift in speciation rate was not accompanied by an acceleration in the rate of morphological evolution in the freshwater crown clade relative to the other species, but is correlated with a reduction in head pores and scales as well as an increase in egg size. Our results challenge the notion that clades always rapidly exploit ecological opportunities in the absence of competing lineages. Instead, we demonstrate that adaptive radiation can experience a slow start before undergoing accelerated diversification and that lineage and phenotypic diversification may be uncoupled in young radiations. [Adaptive radiation; Eleotridae; freshwater; Gobiomorphus; New Zealand.].
© The Author(s) 2021. Published by Oxford University Press, on behalf of the Society of Systematic Biologists.
Figures



References
-
- Alfaro M.E., Faircloth B.C., Harrington R.C., Sorenson L., Friedman M., Thacker C.E., Oliveros C.H., Černý D., Near T.J.. 2018. Explosive diversification of marine fishes at the Cretaceous-Palaeogene boundary. Nature Ecol. Evol. 2:688–696. - PubMed
-
- Allen G.R., Midgley S.H., Allen M.. 2002. Field guide to the freshwater fishes of Australia. Perth: Western Australian Museum. 394 p.
-
- Bell M.A., Foster S.A.. 1994. Introduction to the evolutionary biology of the threespine stickleback. In: Bell M.A., Foster S.A., editors. The evolutionary biology of the threespine stickleback Oxford UK: Oxford University Press. 1:27.
-
- Burress E.D., Wainwright P.C.. 2018. Adaptive radiation in labrid fishes: a central role for functional innovations during 65 My of relentless diversification. Evolution. 73:346–359. - PubMed
-
- Chapple D.G., Ritchie P.A., Daugherty C.H.. 2009. Origin, diversification, and systematics of the New Zealand skink fauna (Reptilia: Scincidae). Mol. Phyl. Evol. 52:470–487. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

