Pancreatitis severity in mice with impaired CFTR function but pancreatic sufficiency is mediated via ductal and inflammatory cells-Not acinar cells
- PMID: 33682322
- PMCID: PMC8107082
- DOI: 10.1111/jcmm.16404
Pancreatitis severity in mice with impaired CFTR function but pancreatic sufficiency is mediated via ductal and inflammatory cells-Not acinar cells
Abstract
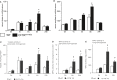
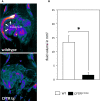
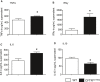
Mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR) are an established risk factor for cystic fibrosis (CF) and chronic pancreatitis. Whereas patients with CF usually develop complete exocrine pancreatic insufficiency, pancreatitis patients with CFTR mutations have mostly preserved exocrine pancreatic function. We therefore used a strain of transgenic mice with significant residual CFTR function (CFTRtm1HGU ) to induce pancreatitis experimentally by serial caerulein injections. Protease activation and necrosis were investigated in isolated acini, disease severity over 24h, pancreatic function by MRI, isolated duct stimulation and faecal chymotrypsin, and leucocyte function by ex vivo lipopolysaccharide (LPS) stimulation. Pancreatic and lung injury were more severe in CFTRtm1HGU but intrapancreatic trypsin and serum enzyme activities higher than in wild-type controls only at 8h, a time interval previously attributed to leucocyte infiltration. CCK-induced trypsin activation and necrosis in acini from CFTRtm1HGU did not differ from controls. Fluid and bicarbonate secretion were greatly impaired, whereas faecal chymotrypsin remained unchanged. LPS stimulation of splenocytes from CFTRtm1HGU resulted in increased INF-γ and IL-6, but decreased IL-10 secretion. CFTR mutations that preserve residual pancreatic function significantly increase the severity of experimental pancreatitis-mostly via impairing duct cell function and a shift towards a pro-inflammatory phenotype, not by rendering acinar cells more susceptible to pathological stimuli.
Keywords: CFTR; acute pancreatitis; ductal cells; inflammatory cells.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures




References
-
- Hoffmeister A, Mayerle J, Beglinger C, et al. English language version of the S3‐consensus guidelines on chronic pancreatitis: Definition, aetiology, diagnostic examinations, medical, endoscopic and surgical management of chronic pancreatitis. Z Für Gastroenterol. 2015;53:1447‐1495. - PubMed
-
- Saluja AK, Dawra RK, Lerch MM, et al. CCK‐JMV‐180, an analog of cholecystokinin, releases intracellular calcium from an inositol trisphosphate‐independent pool in rat pancreatic acini. J Biol Chem. 1992;267:11202‐11207. - PubMed
-
- Halangk W, Krüger B, Ruthenbürger M, et al. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G367‐374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG 203/2-1/4-1/Deutsche Forschungsgemeinschaft
- MA 4115/1-2/3/Deutsche Forschungsgemeinschaft
- SE 2702/2-1/Deutsche Forschungsgemeinschaft
- GRK 1947;A3/Deutsche Forschungsgemeinschaft
- 03IS2061A/Federal Ministry of Education and Research
- 0314107/Federal Ministry of Education and Research
- 01ZZ9603/Federal Ministry of Education and Research
- 01ZZ0103/Federal Ministry of Education and Research
- 01ZZ0403/Federal Ministry of Education and Research
- 03ZIK012/Federal Ministry of Education and Research
- 03zz0921E/Federal Ministry of Education and Research
- 03ZZ0921E/Federal Ministry of Education and Research
- V-630-S-150-2012/132/133/European Union
- ESF/14-BM-A55-0045/16/PePPP centre of excellence MV
- ESF/14-BM-A55-0008/18/EnErGie/P2 Project
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

