Reg1 and Snf1 regulate stress-induced relocalization of protein phosphatase-1 to cytoplasmic granules
- PMID: 33682330
- PMCID: PMC8373691
- DOI: 10.1111/febs.15802
Reg1 and Snf1 regulate stress-induced relocalization of protein phosphatase-1 to cytoplasmic granules
Abstract
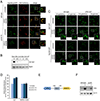
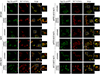
The compartmentalization of cellular function is achieved largely through the existence of membrane-bound organelles. However, recent work suggests a novel mechanism of compartmentalization mediated by membraneless structures that have liquid droplet-like properties and arise through phase separation. Cytoplasmic stress granules (SGs) are the best characterized and are induced by various stressors including arsenite, heat shock, and glucose deprivation. Current models suggest that SGs play an important role in protein homeostasis by mediating reversible translation attenuation. Protein phosphatase-1 (PP1) is a central cellular regulator responsible for most serine/threonine dephosphorylation. Here, we show that upon arsenite stress, PP1's catalytic subunit Glc7 relocalizes to punctate cytoplasmic granules. This altered localization requires PP1's recently described maturation pathway mediated by the multifunctional ATPase Cdc48 and PP1's regulatory subunit Ypi1. Glc7 relocalization is mediated by its regulatory subunit Reg1 and its target Snf1, the AMP-dependent protein kinase. Surprisingly, Glc7 granules are highly specific to arsenite and appear distinct from canonical SGs. Arsenite induces potent translational inhibition, and translational recovery is strongly dependent on Glc7, but independent of Glc7's well-established role in regulating eIF2α. These results suggest a novel form of stress-induced cytoplasmic granule and a new mode of translational control by Glc7.
Keywords: Glc7; Reg1; Snf1; protein phosphatase-1; stress granules.
© 2021 Federation of European Biochemical Societies.
Conflict of interest statement
Conflicts of Interest: The authors declare that they have no conflict of interest.
Figures




References
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Juelicher F & Hyman AA (2009) Germline P granules are liquid droplets that localize by controlled dissolution/ condensation. Science 324, 1729–1732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

