Withdrawing Ixekizumab in Patients With Psoriatic Arthritis Who Achieved Minimal Disease Activity: Results From a Randomized, Double-Blind Withdrawal Study
- PMID: 33682378
- PMCID: PMC8457232
- DOI: 10.1002/art.41716
Withdrawing Ixekizumab in Patients With Psoriatic Arthritis Who Achieved Minimal Disease Activity: Results From a Randomized, Double-Blind Withdrawal Study
Abstract
Objective: To evaluate the effect of withdrawing ixekizumab in patients with psoriatic arthritis (PsA) in whom minimal disease activity (MDA) has been achieved after open-label ixekizumab treatment.
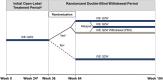
Methods: SPIRIT-P3 was a multicenter, randomized, double-blind withdrawal study of biologic treatment-naive adult patients with PsA who were treated with open-label ixekizumab for 36 weeks (160 mg at week 0, then 80 mg every 2 weeks). Patients in whom MDA was sustained for >3 consecutive months were randomized 1:1, between weeks 36 and 64, to undergo blinded withdrawal of ixekizumab treatment (placebo) or to continue ixekizumab treatment every 2 weeks up to week 104. The primary efficacy end point was time to relapse (loss of MDA) for randomized patients. Patients who experienced a relapse were re-treated with ixekizumab every 2 weeks up to week 104.
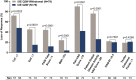
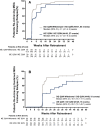
Results: A total of 394 patients were enrolled and received open-label ixekizumab every 2 weeks. Of those patients, 158 (40%) achieved sustained MDA and were randomized to undergo withdrawal of ixekizumab treatment (placebo every 2 weeks; n = 79) or to continue ixekizumab treatment every 2 weeks (n = 79). Disease relapse occurred more rapidly with treatment withdrawal (median 22.3 weeks [95% confidence interval (95% CI) 16.1-28.3]) compared to those who continued treatment with ixekizumab (median not estimable; P < 0.0001). Sixty-seven patients (85%) compared to 30 patients (38%) experienced relapse in the placebo group and the continued treatment group, respectively. Median time to achieving MDA again with re-treatment was 4.1 weeks (95% CI 4.1-4.3); in 64 of 67 patients (96%) who experienced relapse with treatment withdrawal, MDA was achieved again with re-treatment. Safety was consistent with the known safety profile for ixekizumab.
Conclusion: Continued ixekizumab therapy is superior to ixekizumab withdrawal in maintaining low disease activity in biologic treatment-naive patients with PsA. Re-treatment with ixekizumab following a relapse may restore disease control in cases of treatment interruption.
Trial registration: ClinicalTrials.gov NCT02584855.
© 2021 Eli Lilly and Company. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mørk C, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations: data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108–13. - PubMed
-
- Boehncke WH, Menter A. Burden of disease: psoriasis and psoriatic arthritis [review]. Am J Clin Dermatol 2013;14:377–88. - PubMed
-
- Gossec L, Smolen JS, Ramiro S, de Wit M , Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

