The Indiana O'Brien Center for Advanced Renal Microscopic Analysis
- PMID: 33682441
- PMCID: PMC8174807
- DOI: 10.1152/ajprenal.00007.2021
The Indiana O'Brien Center for Advanced Renal Microscopic Analysis
Abstract
The Indiana O'Brien Center for Advanced Microscopic Analysis is a National Institutes of Health (NIH) P30-funded research center dedicated to the development and dissemination of advanced methods of optical microscopy to support renal researchers throughout the world. The Indiana O'Brien Center was founded in 2002 as an NIH P-50 project with the original goal of helping researchers realize the potential of intravital multiphoton microscopy as a tool for understanding renal physiology and pathophysiology. The center has since expanded into the development and implementation of large-scale, high-content tissue cytometry. The advanced imaging capabilities of the center are made available to renal researchers worldwide via collaborations and a unique fellowship program. Center outreach is accomplished through an enrichment core that oversees a seminar series, an informational website, and a biennial workshop featuring hands-on training from members of the Indiana O'Brien Center and imaging experts from around the world.
Keywords: O'Brien Center; fluorescence microscopy; intravital microscopy; kidney.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

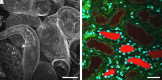
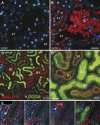
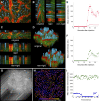



References
-
- Endres BT, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Kamocka MM, McDermott-Roe C, Staruschenko A, Molitoris BA, Geurts AM, Palygin O. Intravital imaging of the kidney in a rat model of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F163–F173, 2017. doi:10.1152/ajprenal.00466.2016. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

