The α-Synuclein Origin and Connectome Model (SOC Model) of Parkinson's Disease: Explaining Motor Asymmetry, Non-Motor Phenotypes, and Cognitive Decline
- PMID: 33682732
- PMCID: PMC8150555
- DOI: 10.3233/JPD-202481
The α-Synuclein Origin and Connectome Model (SOC Model) of Parkinson's Disease: Explaining Motor Asymmetry, Non-Motor Phenotypes, and Cognitive Decline
Abstract
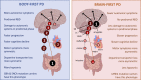
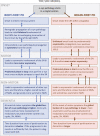
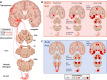
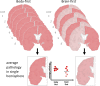
A new model of Parkinson's disease (PD) pathogenesis is proposed, the α-Synuclein Origin site and Connectome (SOC) model, incorporating two aspects of α-synuclein pathobiology that impact the disease course for each patient: the anatomical location of the initial α-synuclein inclusion, and α-synuclein propagation dependent on the ipsilateral connections that dominate connectivity of the human brain. In some patients, initial α-synuclein pathology occurs within the CNS, leading to a brain-first subtype of PD. In others, pathology begins in the peripheral autonomic nervous system, leading to a body-first subtype. In brain-first cases, it is proposed that the first pathology appears unilaterally, often in the amygdala. If α-synuclein propagation depends on connection strength, a unilateral focus of pathology will disseminate more to the ipsilateral hemisphere. Thus, α-synuclein spreads mainly to ipsilateral structures including the substantia nigra. The asymmetric distribution of pathology leads to asymmetric dopaminergic degeneration and motor asymmetry. In body-first cases, the α-synuclein pathology ascends via the vagus to both the left and right dorsal motor nuclei of the vagus owing to the overlapping parasympathetic innervation of the gut. Consequently, the initial α-synuclein pathology inside the CNS is more symmetric, which promotes more symmetric propagation in the brainstem, leading to more symmetric dopaminergic degeneration and less motor asymmetry. At diagnosis, body-first patients already have a larger, more symmetric burden of α-synuclein pathology, which in turn promotes faster disease progression and accelerated cognitive decline. The SOC model is supported by a considerable body of existing evidence and may have improved explanatory power.
Keywords: Parkinson’s disease; alpha-synuclein; autonomic nervous system; connectome; etiology; pathogenesis.
Conflict of interest statement
The author has no conflict of interest to report.
Figures


References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388, 839–840. - PubMed
-
- Goedert M, Masuda-Suzukake M, Falcon B (2017) Like prions: The propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain 140, 266–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

