α-Synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson's disease
- PMID: 33682798
- PMCID: PMC8021121
- DOI: 10.1172/jci.insight.135633
α-Synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson's disease
Abstract
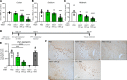
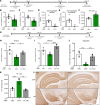
Parkinson's disease (PD) is a prevalent neurodegenerative disease with no approved disease-modifying therapies. Multiplications, mutations, and single nucleotide polymorphisms in the SNCA gene, encoding α-synuclein (aSyn) protein, either cause or increase risk for PD. Intracellular accumulations of aSyn are pathological hallmarks of PD. Taken together, reduction of aSyn production may provide a disease-modifying therapy for PD. We show that antisense oligonucleotides (ASOs) reduce production of aSyn in rodent preformed fibril (PFF) models of PD. Reduced aSyn production leads to prevention and removal of established aSyn pathology and prevents dopaminergic cell dysfunction. In addition, we address the translational potential of the approach through characterization of human SNCA-targeting ASOs that efficiently suppress the human SNCA transcript in vivo. We demonstrate broad activity and distribution of the human SNCA ASOs throughout the nonhuman primate brain and a corresponding decrease in aSyn cerebral spinal fluid (CSF) levels. Taken together, these data suggest that, by inhibiting production of aSyn, it may be possible to reverse established pathology; thus, these data support the development of SNCA ASOs as a potential disease-modifying therapy for PD and related synucleinopathies.
Keywords: Neuroscience; Parkinson disease; Therapeutics.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

