Neurotensin: a neuropeptide induced by hCG in the human and rat ovary during the periovulatory period†
- PMID: 33682882
- PMCID: PMC8485077
- DOI: 10.1093/biolre/ioab036
Neurotensin: a neuropeptide induced by hCG in the human and rat ovary during the periovulatory period†
Abstract
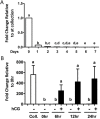
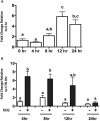
Neurotensin (NTS) is a tridecapeptide that was first characterized as a neurotransmitter in neuronal cells. The present study examined ovarian NTS expression across the periovulatory period in the human and the rat. Women were recruited into this study and monitored by transvaginal ultrasound. The dominant follicle was surgically excised prior to the luteinizing hormone (LH) surge (preovulatory phase) or women were given 250 μg human chorionic gonadotropin (hCG) and dominant follicles collected 12-18 h after hCG (early ovulatory), 18-34 h (late ovulatory), and 44-70 h (postovulatory). NTS mRNA was massively induced during the early and late ovulatory stage in granulosa cells (GCs) (15 000 fold) and theca cells (700 fold). In the rat, hCG also induced Nts mRNA expression in intact ovaries and isolated GCs. In cultured granulosa-luteal cells (GLCs) from IVF patients, NTS expression was induced 6 h after hCG treatment, whereas in cultured rat GCs, NTS increased 4 h after hCG treatment. Cells treated with hCG signaling pathway inhibitors revealed that NTS expression is partially regulated in the human and rat GC by the epidermal-like growth factor pathway. Human GLC, and rat GCs also showed that Nts was regulated by the protein kinase A (PKA) pathway along with input from the phosphotidylinositol 3- kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways. The predominat NTS receptor present in human and rat GCs was SORT1, whereas NTSR1 and NTSR2 expression was very low. Based on NTS actions in other systems, we speculate that NTS may regulate crucial aspects of ovulation such as vascular permeability, inflammation, and cell migration.
Keywords: cumulus oocyte complex; fertility; granulosa cell; neurotensin; ovary; ovulation.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Leeman SE, Carraway RE. Neurotensin: discovery, isolation, characterization, synthesis and possible physiological roles. Ann N Y Acad Sci 1982; 400:1–16. - PubMed
-
- Enjalbert A, Arancibia S, Priam M, Bluet-Pajot MT, Kordon C. Neurotensin stimulation of prolactin secretion in vitro. Neuroendocrinology 1982; 34:95–98. - PubMed
-
- Leeman SE. Substance P and neurotensin: discovery, isolation, chemical characterization and physiological studies. J Exp Biol 1980; 89:193–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

