Integrase Strand Transfer Inhibitor Resistance in Integrase Strand Transfer Inhibitor-Naive Persons
- PMID: 33683148
- PMCID: PMC8665799
- DOI: 10.1089/AID.2020.0261
Integrase Strand Transfer Inhibitor Resistance in Integrase Strand Transfer Inhibitor-Naive Persons
Abstract
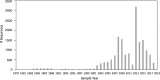
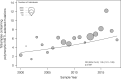
There has been no systematic review of the prevalence of transmitted integrase strand transfer inhibitor (INSTI) resistance. We systematically searched the English-language PubMed database and GenBank to identify studies published since 2010 reporting 50 or more INSTI-naive HIV-1-infected adults undergoing integrase genotyping. We extracted data related to country, sample year, specimen type, sequencing method, and subtype. For studies with sequences in GenBank, we determined the prevalence of three categories of INSTI-associated resistance mutations: (1) nonpolymorphic INSTI-selected drug resistance mutations (DRMs) that we refer to as surveillance DRMs; (2) rarely selected nonpolymorphic INSTI-associated DRMs; and (3) common polymorphic accessory INSTI-selected DRMs. A total of 103 studies met inclusion criteria including 75 studies in GenBank containing integrase sequences from 16,481 INSTI-naive persons. The median sample year was 2013 (interquartile range: 2008-2014). The prevalence of INSTI surveillance DRMs, rarely selected DRMs, and common polymorphic accessory INSTI-selected DRMs were 0.5%, 0.8%, and 6.2%, respectively. There was no association between the presence of nonpolymorphic surveillance DRM and region, sample year, or subtype. Two surveillance DRMs, E138K and R263K occurred in 0.15% and 0.10% of naive sequences, respectively. Several lines of evidence suggested that the 0.5% prevalence of surveillance DRMs partly reflects the cumulative natural occurrence of these mutations in the absence of selective drug pressure. There was an unexplained temporal increase in the proportion of sequences with polymorphic accessory mutations. The prevalence of INSTI-associated surveillance DRMs is low even in regions where INSTIs have been a major component of antiretroviral therapy for several years. The presence of INSTI-associated surveillance DRMs in INSTI-naive persons likely results from actual cases of transmitted INSTI resistance and from a low background level reflecting the cumulative rare natural occurrence of several nonpolymorphic mutations.
Keywords: HIV-1; antiretroviral therapy; drug resistance; integrase; surveillance.
Conflict of interest statement
No competing financial interests exist.
Figures

Similar articles
-
Integrase strand transfer inhibitor (INSTI)-resistance mutations for the surveillance of transmitted HIV-1 drug resistance.J Antimicrob Chemother. 2020 Jan 1;75(1):170-182. doi: 10.1093/jac/dkz417. J Antimicrob Chemother. 2020. PMID: 31617907 Free PMC article.
-
Prevalence of integrase strand transfer inhibitor resistance mutations in antiretroviral-naive HIV-1-infected individuals in Cameroon.J Antimicrob Chemother. 2021 Jan 1;76(1):124-129. doi: 10.1093/jac/dkaa383. J Antimicrob Chemother. 2021. PMID: 32954411
-
Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review.Viruses. 2023 Sep 15;15(9):1932. doi: 10.3390/v15091932. Viruses. 2023. PMID: 37766338 Free PMC article.
-
Prevalence of major INSTI and HIV-1 drug resistance mutations in pre- and antiretroviral-treated patients in Indonesia.Narra J. 2024 Dec;4(3):e1022. doi: 10.52225/narra.v4i3.1022. Epub 2024 Oct 23. Narra J. 2024. PMID: 39816057 Free PMC article.
-
A systematic review of the genetic mechanisms of dolutegravir resistance.J Antimicrob Chemother. 2019 Nov 1;74(11):3135-3149. doi: 10.1093/jac/dkz256. J Antimicrob Chemother. 2019. PMID: 31280314 Free PMC article.
Cited by
-
Prevalence of HIV-1 Natural Polymorphisms and Integrase-Resistance-Associated Mutations in African Children.Viruses. 2023 Feb 16;15(2):546. doi: 10.3390/v15020546. Viruses. 2023. PMID: 36851760 Free PMC article.
-
Pre-Treatment Integrase Inhibitor Resistance and Natural Polymorphisms among HIV-1 Subtype C Infected Patients in Ethiopia.Viruses. 2022 Mar 30;14(4):729. doi: 10.3390/v14040729. Viruses. 2022. PMID: 35458459 Free PMC article.
-
Integrase strand transfer inhibitors resistance-associated mutations in HIV-infected pregnant women.Rev Esp Quimioter. 2024 Dec;37(6):479-485. doi: 10.37201/req/074.2024. Epub 2024 Oct 4. Rev Esp Quimioter. 2024. PMID: 39364596 Free PMC article.
-
Treatment of Advanced HIV in the Modern Era.Drugs. 2025 Jul;85(7):883-909. doi: 10.1007/s40265-025-02181-1. Epub 2025 May 12. Drugs. 2025. PMID: 40354016 Free PMC article. Review.
-
Predicted effects of the introduction of long-acting injectable cabotegravir pre-exposure prophylaxis in sub-Saharan Africa: a modelling study.Lancet HIV. 2023 Apr;10(4):e254-e265. doi: 10.1016/S2352-3018(22)00365-4. Epub 2023 Jan 12. Lancet HIV. 2023. PMID: 36642087 Free PMC article.
References
-
- U.S. Department of Health and Human Services: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2014. Available at https://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf, accessed June 1, 2020.
-
- World Health Organization: Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. HIV Treatment—Interim Guidance. 2018. Available at https://www.who.int/hiv/pub/guidelines/ARV2018update/en/, accessed June 1, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

