Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice
- PMID: 33683198
- PMCID: PMC8060029
- DOI: 10.7554/eLife.51675
Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice
Abstract
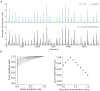
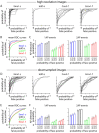
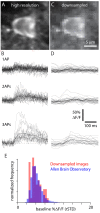
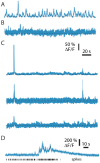
Fluorescent calcium indicators are often used to investigate neural dynamics, but the relationship between fluorescence and action potentials (APs) remains unclear. Most APs can be detected when the soma almost fills the microscope's field of view, but calcium indicators are used to image populations of neurons, necessitating a large field of view, generating fewer photons per neuron, and compromising AP detection. Here, we characterized the AP-fluorescence transfer function in vivo for 48 layer 2/3 pyramidal neurons in primary visual cortex, with simultaneous calcium imaging and cell-attached recordings from transgenic mice expressing GCaMP6s or GCaMP6f. While most APs were detected under optimal conditions, under conditions typical of population imaging studies, only a minority of 1 AP and 2 AP events were detected (often <10% and ~20-30%, respectively), emphasizing the limits of AP detection under more realistic imaging conditions.
Keywords: action potential; calcium imaging; calibration; cell-attached recording; excitatory neurons; genetically encoded calcium indicator; mouse; neuroscience.
Plain language summary
Neurons, the cells that make up the nervous system, transmit information using electrical signals known as action potentials or spikes. Studying the spiking patterns of neurons in the brain is essential to understand perception, memory, thought, and behaviour. One way to do that is by recording electrical activity with microelectrodes. Another way to study neuronal activity is by using molecules that change how they interact with light when calcium binds to them, since changes in calcium concentration can be indicative of neuronal spiking. That change can be observed with specialized microscopes know as two-photon fluorescence microscopes. Using calcium indicators, it is possible to simultaneously record hundreds or even thousands of neurons. However, calcium fluorescence and spikes do not translate one-to-one. In order to interpret fluorescence data, it is important to understand the relationship between the fluorescence signals and the spikes associated with individual neurons. The only way to directly measure this relationship is by using calcium imaging and electrical recording simultaneously to record activity from the same neuron. However, this is extremely challenging experimentally, so this type of data is rare. To shed some light on this, Huang, Ledochowitsch et al. used mice that had been genetically modified to produce a calcium indicator in neurons of the visual cortex and simultaneously obtained both fluorescence measurements and electrical recordings from these neurons. These experiments revealed that, while the majority of time periods containing multi-spike neural activity could be identified using calcium imaging microscopy, on average, less than 10% of isolated single spikes were detectable. This is an important caveat that researchers need to take into consideration when interpreting calcium imaging results. These findings are intended to serve as a guide for interpreting calcium imaging studies that look at neurons in the mammalian brain at the population level. In addition, the data provided will be useful as a reference for the development of activity sensors, and to benchmark and improve computational approaches for detecting and predicting spikes.
© 2021, Huang et al.
Conflict of interest statement
LH, PL, UK, JL, GM, RR, Sd, CK, HZ, MB, JW, LL No competing interests declared
Figures






References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium Indicator for neural activity imaging. Journal of Neuroscience. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. - DOI - PMC - PubMed
-
- Berens P, Freeman J, Deneux T, Chenkov N, McColgan T, Speiser A, Macke JH, Turaga SC, Mineault P, Rupprecht P, Gerhard S, Friedrich RW, Friedrich J, Paninski L, Pachitariu M, Harris KD, Bolte B, Machado TA, Ringach D, Stone J, Rogerson LE, Sofroniew NJ, Reimer J, Froudarakis E, Euler T, Román Rosón M, Theis L, Tolias AS, Bethge M. Community-based benchmarking improves spike rate inference from two-photon calcium imaging data. PLOS Computational Biology. 2018;14:e1006157. doi: 10.1371/journal.pcbi.1006157. - DOI - PMC - PubMed
-
- Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, Mills M, Bosma-Moody A, Siverts LA, Walker M, Graybuck LT, Yao Z, Fong O, Nguyen TN, Garren E, Lenz GH, Chavarha M, Pendergraft J, Harrington J, Hirokawa KE, Harris JA, Nicovich PR, McGraw MJ, Ollerenshaw DR, Smith KA, Baker CA, Ting JT, Sunkin SM, Lecoq J, Lin MZ, Boyden ES, Murphy GJ, da Costa NM, Waters J, Li L, Tasic B, Zeng H. A suite of transgenic driver and reporter mouse lines with enhanced Brain-Cell-Type targeting and functionality. Cell. 2018;174:465–480. doi: 10.1016/j.cell.2018.06.035. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

