Long-term outcomes of R-CEOP show curative potential in patients with DLBCL and a contraindication to anthracyclines
- PMID: 33683338
- PMCID: PMC7948286
- DOI: 10.1182/bloodadvances.2020002982
Long-term outcomes of R-CEOP show curative potential in patients with DLBCL and a contraindication to anthracyclines
Abstract
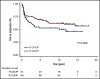
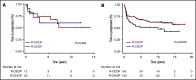
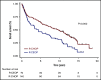
Doxorubicin plays an integral role in the treatment of patients with diffuse large B-cell lymphoma (DLBCL) but can be associated with significant toxicity. Treatment guidelines of British Columbia (BC) Cancer recommend the substitution of etoposide for doxorubicin in standard-dose R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (R-CEOP) for patients who have a contraindication to anthracyclines; however, it is unknown if this compromises treatment outcome. We identified all patients with newly diagnosed DLBCL who were treated in BC with curative intent with R-CEOP (n = 70) within the study period. Outcome in this population was compared with a 2:1 case-matched control group (n = 140) treated with R-CHOP and matched for age, clinical stage, and International Prognostic Index score. The 10-year time to progression and disease-specific survival were not significantly different for patients treated with R-CEOP compared with patients in the R-CHOP control group (53% vs 62% [P = .089] and 58% vs 67% [P = .251], respectively). The 10-year overall survival was lower in the R-CEOP group (30% vs 49%, P = .002), reflecting the impact of underlying comorbidities and frailty of this population. R-CEOP represents a useful treatment alternative for patients with DLBCL and an absolute contraindication to the use of anthracyclines, with curative potential.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.A.M. has served on the advisory board of Roche, Janssen, and Takeda. C.F. received research funding from Roche and Teva and honoraria from Seattle Genetics, Janssen, Amgen, Celgene, AbbVie, and Sanofi. T.N.S. has served on the advisory board of Eli Lilly, Roche, Novartis, and Purdue. L.H.S. discloses honoraria from Abbvie, Celgene, Janssen, and Lundbeck; honoraria from and consultancy for Acerta, Amgen, apobiologix, AstraZeneca, Gilead, Karyopharm, Kite, Merck, Morphosys, Takeda, Teva, and TG Therapeutics; and consultancy for and honoraria and research funding from Roche/Genetech. The remaining authors declare no competing financial interests.
Figures


References
-
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780-2795. - PubMed
-
- Fisher RI, Gaynor ER, Dahlberg S, et al. . Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002-1006. - PubMed
-
- Coiffier B, Lepage E, Briere J, et al. . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. - PubMed
-
- Sehn LH, Donaldson J, Chhanabhai M, et al. . Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. - PubMed
-
- McKelvey EM, Gottlieb JA, Wilson HE, et al. . Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38(4):1484-1493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

