Ocular dominance columns in V1 are more susceptible than associated callosal patches to imbalance of eye input during precritical and critical periods
- PMID: 33683706
- PMCID: PMC8165009
- DOI: 10.1002/cne.25134
Ocular dominance columns in V1 are more susceptible than associated callosal patches to imbalance of eye input during precritical and critical periods
Erratum in
-
Erratum.J Comp Neurol. 2021 Dec;529(17):3845-3863. doi: 10.1002/cne.25222. Epub 2021 Aug 30. J Comp Neurol. 2021. PMID: 34463057 No abstract available.
Abstract
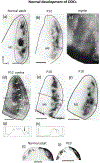
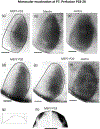
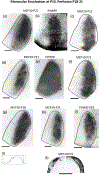
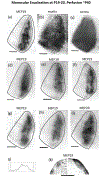
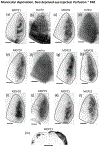
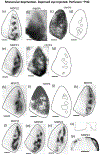
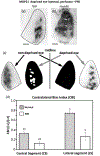
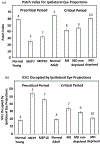
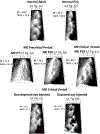
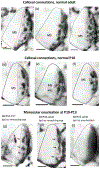
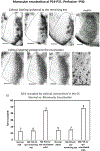
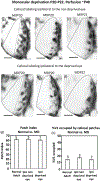
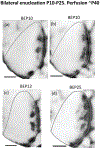
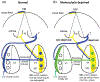
In Long Evans rats, ocular dominance columns (ODCs) in V1 overlap with patches of callosal connections. Using anatomical tracers, we found that ODCs and callosal patches are present at postnatal day 10 (P10), several days before eye opening, and about 10 days before the activation of the critical period for ocular dominance plasticity (~P20). In rats monocularly enucleated at P10 and perfused ~P20, ODCs ipsilateral to the remaining eye desegregated, indicating that rat ODCs are highly susceptible to monocular enucleation during a precritical period. Monocular enucleation during the critical period exerted significant, although smaller, effects. Monocular eye lid suture during the critical period led to a significant expansion of the ipsilateral projection from the nondeprived eye, whereas the contralateral projection invaded into, and intermixed with, ipsilateral ODCs innervated by the deprived eye. We propose that this intermixing allows callosal connections to contribute to the effects of monocular deprivation assessed in the hemisphere ipsilateral to the nondeprived eye. The ipsilateral and contralateral projections from the deprived eye did not undergo significant shrinkage. In contrast, we found that callosal patches are less susceptible to imbalance of eye input. In rats monocularly enucleated during either the precritical or critical periods, callosal patches were maintained in the hemisphere ipsilateral to the remaining eye, but desegregated in the hemisphere ipsilateral to the enucleated orbit. Callosal patches were maintained in rats binocularly enucleated at P10 or later. Similarly, monocular deprivation during the critical period had no significant effect on callosal patches in either hemisphere.
Keywords: Long Evans rats; RRID:RGD 68073; columnar organization; desegregation; eye-specific domains; interhemispheric connections; monocular deprivation; monocular enucleation; primary visual cortex.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
Authors declare no conflict of interest
Figures
References
-
- Aggoun-Zouaoui D, Kiper DC, & Innocenti GM (1996). Growth of callosal terminal arbors in primary visual areas of the cat. European Journal of Neuroscience, 8(6), 1132–1148. - PubMed
-
- Boyd J, & Matsubara J (1994). Tangential organization of callosal connectivity in the cat’s visual cortex. The Journal of Comparative Neurology, 347(2), 197–210. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

