ARID1B/SUB1-activated lncRNA HOXA-AS2 drives the malignant behaviour of hepatoblastoma through regulation of HOXA3
- PMID: 33683826
- PMCID: PMC8034473
- DOI: 10.1111/jcmm.16435
ARID1B/SUB1-activated lncRNA HOXA-AS2 drives the malignant behaviour of hepatoblastoma through regulation of HOXA3
Abstract
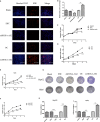
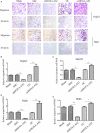
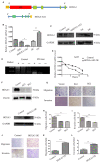
It has been becoming increasingly evident that long non-coding RNAs (lncRNAs) play important roles in various human cancers. However, the biological processes and clinical significance of most lncRNAs in hepatoblastoma (HB) remain unclear. In our previous study, genome-wide analysis with a lncRNA microarray found that lncRNA HOXA-AS2 was up-regulated in HB. Stable transfected cell lines with HOXA-AS2 knockdown or overexpression were constructed in HepG2 and Huh6 cells, respectively. Our data revealed knockdown of HOXA-AS2 increased cell apoptosis and inhibited cell proliferation, migration and invasion in HB. Up-regulation of HOXA-AS2 promoted HB malignant biological behaviours. Mechanistic investigations indicated that HOXA-AS2 was modulated by chromatin remodelling factor ARID1B and transcription co-activator SUB1, thereby protecting HOXA3 from degradation. Therefore, HOXA-AS2 positively regulates HOXA3, which might partly demonstrate the involvement of HOXA3 in HOXA-AS2-mediated HB carcinogenesis. In conclusion, HOXA-AS2 is significantly overexpressed in HB and the ARID1B/HOXA-AS2/HOXA3 axis plays a critical role in HB tumorigenesis and development. These results might provide a potential new target for HB diagnosis and therapy.
Keywords: chromatin remodelling factor; hepatoblastoma; lncRNA; tumorigenesis.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Consent for publication: All the authors give their consent for publication.
Figures




References
-
- Naoe A, Tsuchiya T, Kondo Y, et al. Arctigenin induces apoptosis in human hepatoblastoma cells. Pediatr Surg Int. 2019;35:723‐728. - PubMed
-
- Vishnoi JR, Sasidhar A, Misra S, et al. Hepatoblastoma in a young adult: a rare case report and review of the literature. J Gastrointest Cancer. 2019;51(1):319‐324. - PubMed
-
- Arcay A, Kintrup GT, Gelen MT, Arslan G, Karayalcin B. Hepatoblastoma mimicking hemangioma in labeled red blood cell scintigraphy. Clin Nucl Med. 2019;44:229‐231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

