Macrophages bind LDL using heparan sulfate and the perlecan protein core
- PMID: 33684447
- PMCID: PMC8027565
- DOI: 10.1016/j.jbc.2021.100520
Macrophages bind LDL using heparan sulfate and the perlecan protein core
Abstract
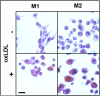
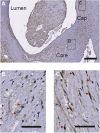
The retention of low-density lipoprotein (LDL) is a key process in the pathogenesis of atherosclerosis and largely mediated via smooth-muscle cell-derived extracellular proteoglycans including the glycosaminoglycan chains. Macrophages can also internalize lipids via complexes with proteoglycans. However, the role of polarized macrophage-derived proteoglycans in binding LDL is unknown and important to advance our understanding of the pathogenesis of atherosclerosis. We therefore examined the identity of proteoglycans, including the pendent glycosaminoglycans, produced by polarized macrophages to gain insight into the molecular basis for LDL binding. Using the quartz crystal microbalance with dissipation monitoring technique, we established that classically activated macrophage (M1)- and alternatively activated macrophage (M2)-derived proteoglycans bind LDL via both the protein core and heparan sulfate (HS) in vitro. Among the proteoglycans secreted by macrophages, we found perlecan was the major protein core that bound LDL. In addition, we identified perlecan in the necrotic core as well as the fibrous cap of advanced human atherosclerotic lesions in the same regions as HS and colocalized with M2 macrophages, suggesting a functional role in lipid retention in vivo. These findings suggest that macrophages may contribute to LDL retention in the plaque by the production of proteoglycans; however, their contribution likely depends on both their phenotype within the plaque and the presence of enzymes, such as heparanase, that alter the secreted protein structure.
Keywords: atherosclerosis; chondroitin sulfate; extracellular matrix; glycosaminoglycan; heparan sulfate; low-density lipoprotein; macrophage; perlecan; proteoglycan; quartz crystal microbalance.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Similar articles
-
Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation.Circ Res. 2008 Jul 3;103(1):43-52. doi: 10.1161/CIRCRESAHA.108.172833. Circ Res. 2008. PMID: 18596265 Free PMC article.
-
Macrophage plasma membrane chondroitin sulfate proteoglycan binds oxidized low-density lipoprotein.Atherosclerosis. 2000 Mar;149(1):5-17. doi: 10.1016/s0021-9150(99)00287-7. Atherosclerosis. 2000. PMID: 10704609
-
Human monocyte-derived macrophages secrete two forms of proteoglycan-macrophage colony-stimulating factor that differ in their ability to bind low density lipoproteins.J Biol Chem. 1998 Jun 26;273(26):15985-92. doi: 10.1074/jbc.273.26.15985. J Biol Chem. 1998. PMID: 9632647
-
Lipoprotein modulation of subendothelial heparan sulfate proteoglycans (perlecan) and atherogenicity.Trends Cardiovasc Med. 2000 Feb;10(2):60-5. doi: 10.1016/s1050-1738(00)00048-7. Trends Cardiovasc Med. 2000. PMID: 11150731 Review.
-
Potential roles of vessel wall heparan sulfate proteoglycans in atherosclerosis.Vascul Pharmacol. 2014 Feb;60(2):49-51. doi: 10.1016/j.vph.2013.12.002. Epub 2013 Dec 12. Vascul Pharmacol. 2014. PMID: 24333941 Review.
Cited by
-
The Human Myofibroblast Marker Xylosyltransferase-I: A New Indicator for Macrophage Polarization.Biomedicines. 2022 Nov 9;10(11):2869. doi: 10.3390/biomedicines10112869. Biomedicines. 2022. PMID: 36359389 Free PMC article.
-
Extracellular matrix in vascular homeostasis and disease.Nat Rev Cardiol. 2025 May;22(5):333-353. doi: 10.1038/s41569-024-01103-0. Epub 2025 Jan 2. Nat Rev Cardiol. 2025. PMID: 39743560 Review.
-
Plasma glycocalyx pattern: a mirror of endothelial damage in chronic kidney disease.Clin Kidney J. 2023 Mar 20;16(8):1278-1287. doi: 10.1093/ckj/sfad051. eCollection 2023 Aug. Clin Kidney J. 2023. PMID: 37529650 Free PMC article.
-
Unravelling the limb regeneration mechanisms of Polypedates maculatus, a sub-tropical frog, by transcriptomics.BMC Genomics. 2023 Mar 16;24(1):122. doi: 10.1186/s12864-023-09205-8. BMC Genomics. 2023. PMID: 36927452 Free PMC article.
-
TNF-α Inhibitors in Combination with MTX Reduce Circulating Levels of Heparan Sulfate/Heparin and Endothelial Dysfunction Biomarkers (sVCAM-1, MCP-1, MMP-9 and ADMA) in Women with Rheumatoid Arthritis.J Clin Med. 2022 Jul 20;11(14):4213. doi: 10.3390/jcm11144213. J Clin Med. 2022. PMID: 35887981 Free PMC article.
References
-
- Hurt-Camejo E., Olsson U., Wiklund O., Bondjers G., Camejo G. Cellular consequences of the association of apoB lipoproteins with proteoglycans. Potential contribution to atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997;17:1011–1017. - PubMed
-
- Camejo G., Lalaguna F., Lopez F., Starosta R. Characterization and properties of a lipoprotein-complexing proteoglycan from human aorta. Atherosclerosis. 1980;35:307–320. - PubMed
-
- Kang H., Lu J., Yang J., Fan Y., Deng X. Interaction of arterial proteoglycans with low density lipoproteins (LDLs): From theory to promising therapeutic approaches. Med. Novel Technology Devices. 2019;3:100016.
-
- Ballinger M.L., Ivey M.E., Osman N., Thomas W.G., Little P.J. Endothelin-1 activates ETA receptors on human vascular smooth muscle cells to yield proteoglycans with increased binding to LDL. Atherosclerosis. 2009;205:451–457. - PubMed
-
- Camejo G., Fager G., Rosengren B., Hurt-Camejo E., Bondjers G. Binding of low density lipoproteins by proteoglycans synthesized by proliferating and quiescent human arterial smooth muscle cells. J. Biol. Chem. 1993;268:14131–14137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

