Optimal design of cluster randomised trials with continuous recruitment and prospective baseline period
- PMID: 33685241
- PMCID: PMC8010895
- DOI: 10.1177/1740774520976564
Optimal design of cluster randomised trials with continuous recruitment and prospective baseline period
Abstract
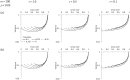
Background: Cluster randomised trials, like individually randomised trials, may benefit from a baseline period of data collection. We consider trials in which clusters prospectively recruit or identify participants as a continuous process over a given calendar period, and ask whether and for how long investigators should collect baseline data as part of the trial, in order to maximise precision.
Methods: We show how to calculate and plot the variance of the treatment effect estimator for different lengths of baseline period in a range of scenarios, and offer general advice.
Results: In some circumstances it is optimal not to include a baseline, while in others there is an optimal duration for the baseline. All other things being equal, the circumstances where it is preferable not to include a baseline period are those with a smaller recruitment rate, smaller intracluster correlation, greater decay in the intracluster correlation over time, or wider transition period between recruitment under control and intervention conditions.
Conclusion: The variance of the treatment effect estimator can be calculated numerically, and plotted against the duration of baseline to inform design. It would be of interest to extend these investigations to cluster randomised trial designs with more than two randomised sequences of control and intervention condition, including stepped wedge designs.
Keywords: Efficient design; group randomised trials; power; sample size.
Conflict of interest statement
Figures





References
-
- Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London: Arnold Publishing, 2000.
-
- Hooper R, Forbes A, Hemming K, et al.. Analysis of cluster randomised trials with an assessment of outcome at baseline. BMJ 2018; 360: k1121. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

