Role of Periostin Expression in Canine Osteosarcoma Biology and Clinical Outcome
- PMID: 33685296
- PMCID: PMC8426451
- DOI: 10.1177/0300985821996671
Role of Periostin Expression in Canine Osteosarcoma Biology and Clinical Outcome
Abstract
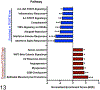
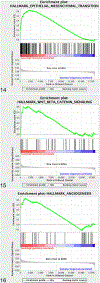
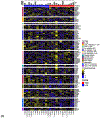
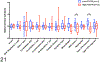
Periostin is a matricellular protein important in regulating bone, tooth, and cardiac development. In pathologic conditions, periostin drives allergic and fibrotic inflammatory diseases and is also overexpressed in certain cancers. Periostin signaling in tumors has been shown to promote angiogenesis, metastasis, and cancer stem cell survival in rodent models, and its overexpression is associated with poor prognosis in human glioblastoma. However, the role of periostin in regulating tumorigenesis of canine cancers has not been evaluated. Given its role in bone development, we sought to evaluate mRNA and protein expression of periostin in canine osteosarcoma (OS) and assess its association with patient outcome. We validated an anti-human periostin antibody cross-reactive to canine periostin via western blot and immunohistochemistry and evaluated periostin expression in microarray data from 49 primary canine OS tumors and 8 normal bone samples. Periostin mRNA was upregulated greater than 40-fold in canine OS tumors compared to normal bone and was significantly correlated with periostin protein expression based on quantitative image analysis. However, neither periostin mRNA nor protein expression were associated with time to metastasis in this cohort. Gene Set Enrichment Analysis demonstrated significant enhancement of pro-tumorigenic pathways including canonical WNT signaling, epithelial-mesenchymal transition, and angiogenesis in periostin-high tumors, while periostin-low tumors demonstrated evidence of heightened antitumor immune responses. Overall, these data identify a novel antibody that can be used as a tool for evaluation of periostin expression in dogs and suggest that investigation of Wnt pathway-targeted drugs in periostin overexpressing canine OS may be a potential therapeutic target.
Keywords: dogs; gene expression; immunohistochemistry; osteosarcoma; periostin.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
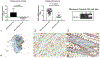
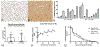

Similar articles
-
HES1, a target of Notch signaling, is elevated in canine osteosarcoma, but reduced in the most aggressive tumors.BMC Vet Res. 2013 Jul 1;9:130. doi: 10.1186/1746-6148-9-130. BMC Vet Res. 2013. PMID: 23816051 Free PMC article.
-
Pro-tumorigenic effects of transforming growth factor beta 1 in canine osteosarcoma.J Vet Intern Med. 2014 May-Jun;28(3):894-904. doi: 10.1111/jvim.12348. Epub 2014 Mar 31. J Vet Intern Med. 2014. PMID: 24684686 Free PMC article.
-
The Association of Endothelin-1 Signaling with Bone Alkaline Phosphatase Expression and Protumorigenic Activities in Canine Osteosarcoma.J Vet Intern Med. 2015 Nov-Dec;29(6):1584-94. doi: 10.1111/jvim.13635. Epub 2015 Oct 1. J Vet Intern Med. 2015. PMID: 26426813 Free PMC article.
-
Role of periostin and its antagonist PNDA-3 in gastric cancer metastasis.World J Gastroenterol. 2015 Mar 7;21(9):2605-13. doi: 10.3748/wjg.v21.i9.2605. World J Gastroenterol. 2015. PMID: 25759527 Free PMC article. Review.
-
Prognostic and predictive biomarkers of canine osteosarcoma.Vet J. 2010 Jul;185(1):28-35. doi: 10.1016/j.tvjl.2010.04.010. Epub 2010 May 20. Vet J. 2010. PMID: 20493743 Review.
Cited by
-
Canine urothelial carcinoma: expression of Periostin in spontaneous canine urothelial carcinoma and its correlation with histological features.Front Vet Sci. 2023 Nov 16;10:1258247. doi: 10.3389/fvets.2023.1258247. eCollection 2023. Front Vet Sci. 2023. PMID: 38076555 Free PMC article.
-
The Recruitment and Immune Suppression Mechanisms of Myeloid-Derived Suppressor Cells and Their Impact on Bone Metastatic Cancer.Cancer Rep (Hoboken). 2025 Feb;8(2):e70044. doi: 10.1002/cnr2.70044. Cancer Rep (Hoboken). 2025. PMID: 39947253 Free PMC article. Review.
References
-
- Bao S, Ouyang G, Bai X, et al.Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5(6):329–339. - PubMed
-
- Ben QW, Zhao Z, Ge SF, et al.Circulating levels of periostin may help identify patients with more aggressive colorectal cancer. Int J Oncol. 2009;34(3):821–828. - PubMed
-
- Bindea G, Mlecnik B, Tosolini M, et al.Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

