App-Based Treatment in Primary Care for Urinary Incontinence: A Pragmatic, Randomized Controlled Trial
- PMID: 33685871
- PMCID: PMC7939722
- DOI: 10.1370/afm.2585
App-Based Treatment in Primary Care for Urinary Incontinence: A Pragmatic, Randomized Controlled Trial
Abstract
Purpose: Electronic application (app)-based treatment is promising for common diseases with good conservative management options, such as urinary incontinence (UI) in women, but its effectiveness compared with usual care is unclear. This study set out to determine if app-based treatment for women with stress, urgency, or mixed UI was noninferior to usual care in the primary care setting.
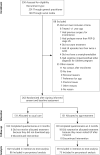
Methods: The URinControl trial is a pragmatic, noninferiority randomized controlled trial in Dutch primary care including adult women with 2 episodes of UI per week. From July 2015 to July 2018, we screened 350 women for eligibility. A stand-alone app-based treatment with pelvic floor muscle and bladder training (URinControl) was compared with usual care according to the Dutch general practitioner guideline for UI treatment. Outcomes measured were change in symptom severity score from baseline to 4 months (primary outcome), impact on disease-specific quality of life, patient-perceived improvement, and number of UI episodes. Noninferiority (<1.5 points) was assessed with linear regression analysis.
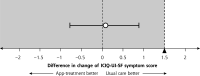
Results: A total of 262 eligible women were randomized equally; 195 of them had follow-up through 4 months. The change in symptom severity with app-based treatment (-2.16 points; 95% CI, -2.67 to -1.65) was noninferior to that with usual care (-2.56 points; 95% CI, -3.28 to -1.84), with a mean difference of 0.058 points (95% CI, -0.776 to 0.891) between groups. Neither treatment was superior to the other, and both groups showed improvements in outcome measures after treatment.
Conclusions: App-based treatment for women with UI was at least as effective as usual care in the primary care setting. As such, app-based treatments, with their potential advantages of privacy, accessibility, and lower cost, may provide women with a good alternative to consultation.
Keywords: app; eHealth; general practice; medical informatics; noninferiority; practice-based research; pragmatic; primary care; self-management; urinary incontinence; women’s health.
© 2021 Annals of Family Medicine, Inc.
Figures
Comment in
-
Self-Directed Technology to Improve Urinary Symptoms.Ann Fam Med. 2021 Mar-Apr;19(2):100-101. doi: 10.1370/afm.2659. Ann Fam Med. 2021. PMID: 33685870 Free PMC article. No abstract available.
References
-
- Loohuis A, Chavannes N. Medical apps; Care for the future? [in Dutch]. Huisarts Wet. 2017; 60(9): 440-443.
-
- Asklund I, Nyström E, Sjöström M, Umefjord G, Stenlund H, Samuelsson E. Mobile app for treatment of stress urinary incontinence: a randomized controlled trial. Neurourol Urodyn. 2017; 36(5): 1369-1376. - PubMed
-
- Araujo CC, Marques ADA, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 10.1097/SPV.0000000000000670. Published online ahead of print January 8, 2019. Accessed Feb 1, 2019. - DOI - PubMed
-
- Abrams P, Andersson K-E, Apostolidis A, et al. . Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol Urodyn. 2018; 37(7): 2271–2272. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
