How do established developmental risk-factors for schizophrenia change the way the brain develops?
- PMID: 33686066
- PMCID: PMC7940420
- DOI: 10.1038/s41398-021-01273-2
How do established developmental risk-factors for schizophrenia change the way the brain develops?
Abstract
The recognition that schizophrenia is a disorder of neurodevelopment is widely accepted. The original hypothesis was coined more than 30 years ago and the wealth of supportive epidemiologically data continues to grow. A number of proposals have been put forward to suggest how adverse early exposures in utero alter the way the adult brain functions, eventually producing the symptoms of schizophrenia. This of course is extremely difficult to study in developing human brains, so the bulk of what we know comes from animal models of such exposures. In this review, I will summarise the more salient features of how the major epidemiologically validated exposures change the way the brain is formed leading to abnormal function in ways that are informative for schizophrenia symptomology. Surprisingly few studies have examined brain ontogeny from embryo to adult in such models. However, where there is longitudinal data, various convergent mechanisms are beginning to emerge involving stress and immune pathways. There is also a surprisingly consistent alteration in how very early dopamine neurons develop in these models. Understanding how disparate epidemiologically-validated exposures may produce similar developmental brain abnormalities may unlock convergent early disease-related pathways/processes.
Conflict of interest statement
The author declares no competing interests.
Figures
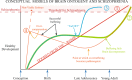
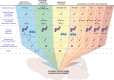
 Increased exposure validated in developing brain/placenta.
Increased exposure validated in developing brain/placenta.  Weak evidence. X No effect. ? Not studied.
Weak evidence. X No effect. ? Not studied.  Altered DNA methylation.
Altered DNA methylation.  Altered Histone acetylation/methylation.
Altered Histone acetylation/methylation.  Increased micro-RNA.
Increased micro-RNA.References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

