A stalled-ribosome rescue factor Pth3 is required for mitochondrial translation against antibiotics in Saccharomyces cerevisiae
- PMID: 33686140
- PMCID: PMC7940416
- DOI: 10.1038/s42003-021-01835-6
A stalled-ribosome rescue factor Pth3 is required for mitochondrial translation against antibiotics in Saccharomyces cerevisiae
Abstract
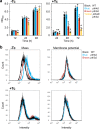
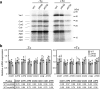
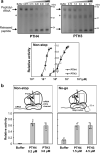
Mitochondrial translation appears to involve two stalled-ribosome rescue factors (srRFs). One srRF is an ICT1 protein from humans that rescues a "non-stop" type of mitochondrial ribosomes (mitoribosomes) stalled on mRNA lacking a stop codon, while the other, C12orf65, reportedly has functions that overlap with those of ICT1; however, its primary role remains unclear. We herein demonstrated that the Saccharomyces cerevisiae homolog of C12orf65, Pth3 (Rso55), preferentially rescued antibiotic-dependent stalled mitoribosomes, which appear to represent a "no-go" type of ribosomes stalled on intact mRNA. On media containing a non-fermentable carbon source, which requires mitochondrial gene expression, respiratory growth was impaired significantly more by the deletion of PTH3 than that of the ICT1 homolog PTH4 in the presence of antibiotics that inhibit mitochondrial translation, such as tetracyclines and macrolides. Additionally, the in organello labeling of mitochondrial translation products and quantification of mRNA levels by quantitative RT-PCR suggested that in the presence of tetracycline, the deletion of PTH3, but not PTH4, reduced the protein expression of all eight mtDNA-encoded genes at the post-transcriptional or translational level. These results indicate that Pth3 can function as a mitochondrial srRF specific for ribosomes stalled by antibiotics and plays a role in antibiotic resistance in fungi.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

