3D-microtissue derived secretome as a cell-free approach for enhanced mineralization of scaffolds in the chorioallantoic membrane model
- PMID: 33686145
- PMCID: PMC7940489
- DOI: 10.1038/s41598-021-84123-x
3D-microtissue derived secretome as a cell-free approach for enhanced mineralization of scaffolds in the chorioallantoic membrane model
Abstract
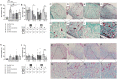
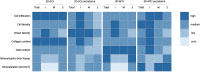
Bone regeneration is a complex process and the clinical translation of tissue engineered constructs (TECs) remains a challenge. The combination of biomaterials and mesenchymal stem cells (MSCs) may enhance the healing process through paracrine effects. Here, we investigated the influence of cell format in combination with a collagen scaffold on key factors in bone healing process, such as mineralization, cell infiltration, vascularization, and ECM production. MSCs as single cells (2D-SCs), assembled into microtissues (3D-MTs) or their corresponding secretomes were combined with a collagen scaffold and incubated on the chicken embryo chorioallantoic membrane (CAM) for 7 days. A comprehensive quantitative analysis was performed on a cellular level by histology and by microcomputed tomography (microCT). In all experimental groups, accumulation of collagen and glycosaminoglycan within the scaffold was observed over time. A pronounced cell infiltration and vascularization from the interface to the surface region of the CAM was detected. The 3D-MT secretome showed a significant mineralization of the biomaterial using microCT compared to all other conditions. Furthermore, it revealed a homogeneous distribution pattern of mineralization deposits in contrast to the cell-based scaffolds, where mineralization was only at the surface. Therefore, the secretome of MSCs assembled into 3D-MTs may represent an interesting therapeutic strategy for a next-generation bone healing concept.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

