Pharmacological targeting of host chaperones protects from pertussis toxin in vitro and in vivo
- PMID: 33686161
- PMCID: PMC7940712
- DOI: 10.1038/s41598-021-84817-2
Pharmacological targeting of host chaperones protects from pertussis toxin in vitro and in vivo
Abstract
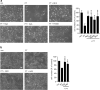
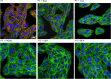
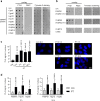
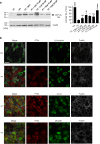
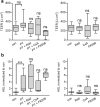
Whooping cough is caused by Bordetella pertussis that releases pertussis toxin (PT) which comprises enzyme A-subunit PTS1 and binding/transport B-subunit. After receptor-mediated endocytosis, PT reaches the endoplasmic reticulum from where unfolded PTS1 is transported to the cytosol. PTS1 ADP-ribosylates G-protein α-subunits resulting in increased cAMP signaling. Here, a role of target cell chaperones Hsp90, Hsp70, cyclophilins and FK506-binding proteins for cytosolic PTS1-uptake is demonstrated. PTS1 specifically and directly interacts with chaperones in vitro and in cells. Specific pharmacological chaperone inhibition protects CHO-K1, human primary airway basal cells and a fully differentiated airway epithelium from PT-intoxication by reducing intracellular PTS1-amounts without affecting cell binding or enzyme activity. PT is internalized by human airway epithelium secretory but not ciliated cells and leads to increase of apical surface liquid. Cyclophilin-inhibitors reduced leukocytosis in infant mouse model of pertussis, indicating their promising potential for developing novel therapeutic strategies against whooping cough.
Conflict of interest statement
The authors declare no competing interests.
Figures


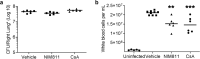
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

