RGS12 is a novel tumor suppressor in osteosarcoma that inhibits YAP-TEAD1-Ezrin signaling
- PMID: 33686240
- PMCID: PMC8694668
- DOI: 10.1038/s41388-020-01599-z
RGS12 is a novel tumor suppressor in osteosarcoma that inhibits YAP-TEAD1-Ezrin signaling
Abstract
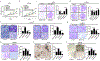
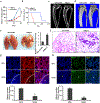
Osteosarcoma (OS) is the most common primary malignancy of the bone that predominantly affects children and adolescents. Hippo pathway is a crucial regulator of organ size and tumorigenesis. However, how Hippo pathway regulates the occurrence of osteosarcoma is largely unknown. Here, we reported the regulator of G protein signaling protein 12 (RGS12) is a novel Hippo pathway regulator and tumor suppressor of osteosarcoma. Depletion of Rgs12 promotes osteosarcoma progression and lung metastasis in an orthotopic xenograft mouse model. Our data showed that the knockdown of RGS12 upregulates Ezrin expression through promoting the GNA12/13-RhoA-YAP pathway. Moreover, RGS12 negatively regulates the transcriptional activity of YAP/TEAD1 complex through its PDZ domain function to inhibit the expression and function of the osteosarcoma marker Ezrin. PDZ domain peptides of RGS12 can inhibit the development of intratibial tumor and lung metastases. Collectively, this study identifies that the RGS12 is a novel tumor suppressor in osteosarcoma through inhibiting YAP-TEAD1-Ezrin signaling pathway and provides a proof of principle that targeting RGS12 may be a therapeutic strategy for osteosarcoma.
Conflict of interest statement
Conflict of interest
The authors declare no competing interests.
Figures


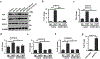



References
-
- Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer 2014; 14: 722–735. - PubMed
-
- Tang QL, Lu JC, Zou CY, Shao Y, Chen Y, Narala S et al. CDH4 is a novel determinant of osteosarcoma tumorigenesis and metastasis. Oncogene 2018; 37: 3617–3630. - PubMed
-
- Harvey KF, Zhang XM, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

