Early sedation with dexmedetomidine in ventilated critically ill patients and heterogeneity of treatment effect in the SPICE III randomised controlled trial
- PMID: 33686482
- PMCID: PMC7939103
- DOI: 10.1007/s00134-021-06356-8
Early sedation with dexmedetomidine in ventilated critically ill patients and heterogeneity of treatment effect in the SPICE III randomised controlled trial
Abstract
Purpose: To quantify potential heterogeneity of treatment effect (HTE), of early sedation with dexmedetomidine (DEX) compared with usual care, and identify patients who have a high probability of lower or higher 90-day mortality according to age, and other identified clusters.
Methods: Bayesian analysis of 3904 critically ill adult patients expected to receive invasive ventilation > 24 h and enrolled in a multinational randomized controlled trial comparing early DEX with usual care sedation.
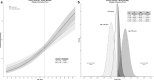
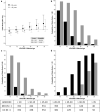
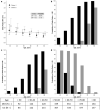
Results: HTE was assessed according to age and clusters (based on 12 baseline characteristics) using a Bayesian hierarchical models. DEX was associated with lower 90-day mortality compared to usual care in patients > 65 years (odds ratio [OR], 0.83 [95% credible interval [CrI] 0.68-1.00], with 97.7% probability of reduced mortality across broad categories of illness severity. Conversely, the probability of increased mortality in patients ≤ 65 years was 98.5% (OR 1.26 [95% CrI 1.02-1.56]. Two clusters were identified: cluster 1 (976 patients) mostly operative, and cluster 2 (2346 patients), predominantly non-operative. There was a greater probability of benefit with DEX in cluster 1 (OR 0.86 [95% CrI 0.65-1.14]) across broad categories of age, with 86.4% probability that DEX is more beneficial in cluster 1 than cluster 2.
Conclusion: In critically ill mechanically ventilated patients, early sedation with dexmedetomidine exhibited a high probability of reduced 90-day mortality in older patients regardless of operative or non-operative cluster status. Conversely, a high probability of increased 90-day mortality was observed in younger patients of non-operative status. Further studies are needed to confirm these findings.
Keywords: Critically ill; Dexmedetomidine; Mechanical ventilation; Mortality; Sedation.
Conflict of interest statement
YS declares unrestricted research and educational Grant support from Pfizer (Hospira Inc IL) USA, Orion Pharma—Helsinki Finland in support of the SPICE Program. Speaker honorarium and travel reimbursements for educational symposia from Pfizer and Orion. MCR declares unrestricted research and educational grant support from Pfizer (Hospira Inc.—Melbourne) Australia. All authors filed in the COI ICMJE form.
Figures
Comment in
-
[Sedation of critically ill patients with dexmedetomidine].Anaesthesiologie. 2023 Mar;72(3):209-211. doi: 10.1007/s00101-022-01244-2. Epub 2023 Jan 4. Anaesthesiologie. 2023. PMID: 36598530 German. No abstract available.
References
-
- Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. - DOI - PubMed
-
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. - DOI - PubMed
-
- Shehabi Y, Forbes AB, Arabi Y, Bass F, Bellomo R, Kadiman S, Howe BD, McArthur C, Reade MC, Seppelt I, et al. The SPICE III study protocol and analysis plan: a randomised trial of early goal-directed sedation compared with standard care in mechanically ventilated patients. Crit Care Resusc. 2017;19(4):318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

