Systematic stability testing of insulins as representative biopharmaceuticals using ATR FTIR-spectroscopy with focus on quality assurance
- PMID: 33686847
- PMCID: PMC7939270
- DOI: 10.1117/1.JBO.26.4.043007
Systematic stability testing of insulins as representative biopharmaceuticals using ATR FTIR-spectroscopy with focus on quality assurance
Abstract
Significance: Bioactive proteins represent the most important component class in biopharmaceutical products for therapeutic applications. Their production is most often biotechnologically realized by genetically engineered microorganisms. For the quality assurance of insulins as representatives of life-saving pharmaceuticals, analytical methods are required that allow more than total protein quantification in vials or batches. Chemical and physical factors such as unstable temperatures or shear rate exposure under storage can lead to misfolding, nucleation, and subsequent fibril forming of the insulins. The assumption is valid that these processes go parallel with a decrease in bioactivity.
Aim: Infrared (IR) spectroscopy has been successfully utilized for secondary structure analysis in cases of protein misfolding and fibril formation.
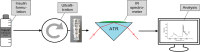
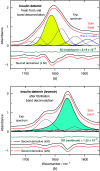
Approach: A reliable method for the quantification of the secondary structure changes has been developed using insulin dry-film Fourier-transform IR spectroscopy in combination with the attenuated total reflection (ATR) technique and subsequent data analyses such as band-shift determination, spectral band deconvolution, and principal component analysis.
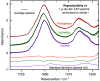
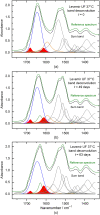
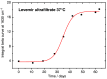
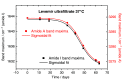
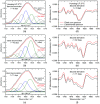
Results: A systematic study of insulin spectra was carried out on model insulin specimens, available either as original formulations or as hormones purified by ultrafiltration. Insulin specimens were stored at different temperatures, i.e., 0°C, 20°C, and 37°C, respectively, for up to three months. Weekly ATR-measurements allowed the monitoring of hormone secondary structure changes, which are supposed to be negatively correlated with insulin bioactivity.
Conclusions: It could be shown that IR-ATR spectroscopy offers a fast and reliable analytical method for the determination of secondary structural changes within insulin molecules, as available in pharmaceutical insulin formulations and therefore challenges internationally established measurement techniques for quality control regarding time, costs, and effort of analysis.
Keywords: dry-film attenuated total reflection spectroscopy; infrared spectroscopy; insulin quality monitoring; point-of-care diagnostics; protein secondary structure analysis.
Figures





References
-
- Santos C., McCaig L., “An executive summary—USP general chapter <1049.1> design of stability studies for biotechnology product development and lifecycle management,” BioPharm International October issue (2019).
-
- Thomas F., “Stability testing: the crucial development step,” Pharm. Technol. Europe 32(3), 28–31 (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

