Reoperative Mitral Surgery Versus Transcatheter Mitral Valve Replacement: A Systematic Review
- PMID: 33686870
- PMCID: PMC8174229
- DOI: 10.1161/JAHA.120.019854
Reoperative Mitral Surgery Versus Transcatheter Mitral Valve Replacement: A Systematic Review
Abstract
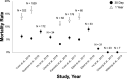
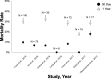
Bioprosthetic mitral structural valve degeneration and failed mitral valve repair (MVr) have traditionally been treated with reoperative mitral valve surgery. Transcatheter mitral valve-in-valve (MVIV) and valve-in-ring (MVIR) replacement are now feasible, but data comparing these approaches are lacking. We sought to compare the outcomes of (1) reoperative mitral valve replacement (redo-MVR) and MVIV for structural valve degeneration, and (2) reoperative mitral valve repair (redo-MVr) or MVR and MVIR for failed MVr. A literature search of PubMed, Embase, and the Cochrane Library was conducted up to July 31, 2020. Thirty-two studies involving 25 832 patients were included. Redo-MVR was required in ≈35% of patients after index surgery at 10 years, with 5% to 15% 30-day mortality. MVIV resulted in >95% procedural success with 30-day and 1-year mortality of 0% to 8% and 11% to 16%, respectively. Recognized complications included left ventricular outflow tract obstruction (0%-6%), valve migration (0%-9%), and residual regurgitation (0%-6%). Comparisons of redo-MVR and MVIV showed no statistically significant differences in mortality (11.3% versus 11.9% at 1 year, P=0.92), albeit higher rates of major bleeding and arrhythmias with redo-MVR. MVIR resulted in 0% to 34% mortality at 1 year, whereas both redo-MVr and MVR for failed repairs were performed with minimal mortality and durable long-term results. MVIV is therefore a viable alternative to redo-MVR for structural valve degeneration, whereas redo-MVr or redo-MVR is preferred for failed MVr given the suboptimal results with MVIR. However, not all patients will be candidates for MVIV/MVIR because anatomical restrictions may preclude transcatheter options from adequately addressing the underlying pathology.
Keywords: redo mitral valve repair; reoperative mitral valve replacement; transcatheter mitral valve replacement; valve‐in‐ring; valve‐in‐valve.
Conflict of interest statement
Dr Tang is a physician proctor for Medtronic and a consultant for Abbott Structural Heart, Medtronic and W.L. Gore & Associates. Dr Kaneko is a speaker for Abbott Structural Heart and Baylis Medical, a consultant for 4C Medical, and has served as a proctor and educator for Edwards Lifesciences and Medtronic. Dr Bapat has served as a consultant for Medtronic, Edwards Lifesciences, 4C Medical, and Boston Scientific. Dr Bhatt discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News,
Figures


Similar articles
-
Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry.Circ Cardiovasc Interv. 2020 Mar;13(3):e008425. doi: 10.1161/CIRCINTERVENTIONS.119.008425. Epub 2020 Mar 6. Circ Cardiovasc Interv. 2020. PMID: 32138529
-
5-Year Prospective Evaluation of Mitral Valve-in-Valve, Valve-in-Ring, and Valve-in-MAC Outcomes: MITRAL Trial Final Results.JACC Cardiovasc Interv. 2023 Sep 25;16(18):2211-2227. doi: 10.1016/j.jcin.2023.06.041. JACC Cardiovasc Interv. 2023. PMID: 37758379
-
Redo mitral valve surgery following prior mitral valve repair.J Card Surg. 2018 Dec;33(12):772-777. doi: 10.1111/jocs.13944. Epub 2018 Dec 12. J Card Surg. 2018. PMID: 30548701
-
Transcatheter Transseptal Mitral Valve-in-Valve Replacement: An Early Australian Case Series and Literature Review.Heart Lung Circ. 2020 Jun;29(6):921-930. doi: 10.1016/j.hlc.2019.07.010. Epub 2019 Aug 22. Heart Lung Circ. 2020. PMID: 31526680 Review.
-
Valve-in-valve transcatheter mitral valve replacement versus redo-surgical mitral valve replacement for degenerated bioprosthetic mitral valves: A systematic review and meta-analysis.Int J Cardiol. 2024 Nov 15;415:132448. doi: 10.1016/j.ijcard.2024.132448. Epub 2024 Aug 15. Int J Cardiol. 2024. PMID: 39153510
Cited by
-
Transcatheter mitral valve replacement versus redo surgery for mitral prosthesis failure: A systematic review and meta-analysis.Front Cardiovasc Med. 2023 Jan 18;9:1058576. doi: 10.3389/fcvm.2022.1058576. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36741847 Free PMC article.
-
Redo hybrid mitral valve-in-valve for early structural valve degeneration: Pearls and pitfalls of a novel technique.JTCVS Tech. 2023 Apr 19;19:41-42. doi: 10.1016/j.xjtc.2023.03.025. eCollection 2023 Jun. JTCVS Tech. 2023. PMID: 37324357 Free PMC article. No abstract available.
-
Mitral surgical redo versus transapical transcatheter mitral valve implantation.PLoS One. 2021 Aug 25;16(8):e0256569. doi: 10.1371/journal.pone.0256569. eCollection 2021. PLoS One. 2021. PMID: 34432834 Free PMC article.
-
2021 ESC/EACTS Guidelines for the management of valvular heart disease.EuroIntervention. 2022 Feb 4;17(14):e1126-e1196. doi: 10.4244/EIJ-E-21-00009. EuroIntervention. 2022. PMID: 34931612 Free PMC article. No abstract available.
-
Mitral Valve Annuloplasty Failure and Percutaneous Treatment Options.Curr Cardiol Rep. 2021 Aug 19;23(10):140. doi: 10.1007/s11886-021-01574-4. Curr Cardiol Rep. 2021. PMID: 34410525 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

