Technologies for Diabetes Self-Monitoring: A Scoping Review and Assessment Using the REASSURED Criteria
- PMID: 33686875
- PMCID: PMC9264435
- DOI: 10.1177/1932296821997909
Technologies for Diabetes Self-Monitoring: A Scoping Review and Assessment Using the REASSURED Criteria
Abstract
Background: Self-management is an important pillar for diabetes control and to achieve it, glucose self-monitoring devices are needed. Currently, there exist several different devices in the market and many others are being developed. However, whether these devices are suitable to be used in resource constrained settings is yet to be evaluated.
Aims: To assess existing glucose monitoring tools and also those in development against the REASSURED which have been previously used to evaluate diagnostic tools for communicable diseases.
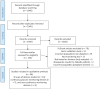
Methods: We conducted a scoping review by searching PubMed for peer-review articles published in either English, Spanish or Portuguese in the last 5 years. We selected papers including information about devices used for self-monitoring and tested on humans with diabetes; then, the REASSURED criteria were used to assess them.
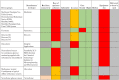
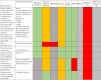
Results: We found a total of 7 continuous glucose monitoring device groups, 6 non-continuous, and 6 devices in development. Accuracy varied between devices and most of them were either invasive or minimally invasive. Little to no evidence is published around robustness, affordability and delivery to those in need. However, when reviewing publicly available prices, none of the devices would be affordable for people living in low- and middle-income countries.
Conclusions: Available devices cannot be considered adapted for use in self-monitoring in resource constraints settings. Further studies should aim to develop less-invasive devices that do not require a large set of components. Additionally, we suggest some improvement in the REASSURED criteria such as the inclusion of patient-important outcomes to increase its appropriateness to assess non-communicable diseases devices.
Keywords: diabetes; monitoring devices; scoping review; self-monitoring.
Conflict of interest statement
Figures
Similar articles
-
User requirements for non-invasive and minimally invasive glucose self-monitoring devices in low-income and middle-income countries: a qualitative study in Kyrgyzstan, Mali, Peru and Tanzania.BMJ Open. 2024 Feb 17;14(2):e076685. doi: 10.1136/bmjopen-2023-076685. BMJ Open. 2024. PMID: 38367964 Free PMC article.
-
Data-driven modeling and prediction of blood glucose dynamics: Machine learning applications in type 1 diabetes.Artif Intell Med. 2019 Jul;98:109-134. doi: 10.1016/j.artmed.2019.07.007. Epub 2019 Jul 26. Artif Intell Med. 2019. PMID: 31383477 Review.
-
Leveraging advances in diabetes technologies in primary care: a narrative review.Ann Med. 2021 Dec;53(1):805-816. doi: 10.1080/07853890.2021.1931427. Ann Med. 2021. PMID: 34184589 Free PMC article. Review.
-
Blood glucose monitoring- an overview of current and future non-invasive devices.Diabetes Metab Syndr. 2020 Sep-Oct;14(5):739-751. doi: 10.1016/j.dsx.2020.05.016. Epub 2020 May 22. Diabetes Metab Syndr. 2020. PMID: 32497964 Review.
-
The Progress of Glucose Monitoring-A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors.Sensors (Basel). 2019 Feb 15;19(4):800. doi: 10.3390/s19040800. Sensors (Basel). 2019. PMID: 30781431 Free PMC article. Review.
Cited by
-
Efficacy of quercetin-like compounds from the mistletoe plant of Dendrophthoe pentandra L. Miq, as oral random blood sugar lowering treatment in diabetic rats.Vet Q. 2024 Dec;44(1):1-14. doi: 10.1080/01652176.2024.2372090. Epub 2024 Jun 29. Vet Q. 2024. PMID: 38943615 Free PMC article.
-
User requirements for non-invasive and minimally invasive glucose self-monitoring devices in low-income and middle-income countries: a qualitative study in Kyrgyzstan, Mali, Peru and Tanzania.BMJ Open. 2024 Feb 17;14(2):e076685. doi: 10.1136/bmjopen-2023-076685. BMJ Open. 2024. PMID: 38367964 Free PMC article.
-
Nanoplasmonic Strip Test for Salivary Glucose Monitoring.Nanomaterials (Basel). 2021 Dec 29;12(1):105. doi: 10.3390/nano12010105. Nanomaterials (Basel). 2021. PMID: 35010055 Free PMC article.
References
-
- Saeedi P, Petersohn I, Salpea P, et al.. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. - PubMed
-
- World Health Organization (WHO). Noncommunicable diseases: childhood overweight and obesity. 2020. Accessed December 12, 2020. https://www.who.int/news-room/q-a-detail/noncommunicable-diseases-childh...
-
- World Health Organization (WHO). Essential medicines and basic health technologies for noncommunicable diseases: towards a set of actions to improve equitable access in Member States. 2015. July.
-
- World Health Organization (WHO). Global action plan for the prevention and control of noncommunicable diseases 2013-2020. 2013. May.
-
- World Health Organization (WHO). Package of essential noncommunicable (PEN) disease interventions for primary health care in low-resource settings. 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

