Penicillium chrysogenum polypeptide extract protects tobacco plants from tobacco mosaic virus infection through modulation of ABA biosynthesis and callose priming
- PMID: 33687058
- PMCID: PMC8096601
- DOI: 10.1093/jxb/erab102
Penicillium chrysogenum polypeptide extract protects tobacco plants from tobacco mosaic virus infection through modulation of ABA biosynthesis and callose priming
Abstract
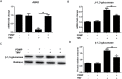
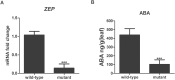
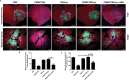
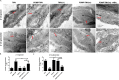
The polypeptide extract of the dry mycelium of Penicillium chrysogenum (PDMP) can protect tobacco plants from tobacco mosaic virus (TMV), although the mechanism underlying PDMP-mediated TMV resistance remains unknown. In our study, we analysed a potential mechanism via RNA sequencing (RNA-seq) and found that the abscisic acid (ABA) biosynthetic pathway and β-1,3-glucanase, a callose-degrading enzyme, might play an important role in PDMP-induced priming of resistance to TMV. To test our hypothesis, we successfully generated a Nicotiana benthamiana ABA biosynthesis mutant and evaluated the role of the ABA pathway in PDMP-induced callose deposition during resistance to TMV infection. Our results suggested that PDMP can induce callose priming to defend against TMV movement. PDMP inhibited TMV movement by increasing callose deposition around plasmodesmata, but this phenomenon did not occur in the ABA biosynthesis mutant; moreover, these effects of PDMP on callose deposition could be rescued by treatment with exogenous ABA. Our results suggested that callose deposition around plasmodesmata in wild-type plants is mainly responsible for the restriction of TMV movement during the PDMP-induced defensive response to TMV infection, and that ABA biosynthesis apparently plays a crucial role in PDMP-induced callose priming for enhancing defence against TMV.
Keywords: N. benthamiana; ABA; PDMP; callose; infection; plasmodesmata; priming; tobacco mosaic virus (TMV).
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Similar articles
-
Nicotiana benthamiana Class 1 Reversibly Glycosylated Polypeptides Suppress Tobacco Mosaic Virus Infection.Int J Mol Sci. 2023 Aug 16;24(16):12843. doi: 10.3390/ijms241612843. Int J Mol Sci. 2023. PMID: 37629021 Free PMC article.
-
iTRAQ-based analysis of leaf proteome identifies important proteins in secondary metabolite biosynthesis and defence pathways crucial to cross-protection against TMV.J Proteomics. 2019 Mar 30;196:42-56. doi: 10.1016/j.jprot.2019.02.002. Epub 2019 Feb 3. J Proteomics. 2019. PMID: 30726703
-
Salicylic acid treatment and expression of an RNA-dependent RNA polymerase 1 transgene inhibit lethal symptoms and meristem invasion during tobacco mosaic virus infection in Nicotiana benthamiana.BMC Plant Biol. 2016 Jan 13;16:15. doi: 10.1186/s12870-016-0705-8. BMC Plant Biol. 2016. PMID: 26757721 Free PMC article.
-
A fungal protease named AsES triggers antiviral immune responses and effectively restricts virus infection in arabidopsis and Nicotiana benthamiana plants.Ann Bot. 2022 Apr 13;129(5):593-606. doi: 10.1093/aob/mcac013. Ann Bot. 2022. PMID: 35134835 Free PMC article.
-
The knowns and unknowns of callose biosynthesis in terrestrial plants.Carbohydr Res. 2024 Apr;538:109103. doi: 10.1016/j.carres.2024.109103. Epub 2024 Mar 28. Carbohydr Res. 2024. PMID: 38555659 Review.
Cited by
-
Priming seeds for the future: Plant immune memory and application in crop protection.Front Plant Sci. 2022 Jul 29;13:961840. doi: 10.3389/fpls.2022.961840. eCollection 2022. Front Plant Sci. 2022. PMID: 35968080 Free PMC article. Review.
-
Genome-Wide Identification and Characterization of the Pirin Gene Family in Nicotiana benthamiana.Genes (Basel). 2025 Jan 22;16(2):121. doi: 10.3390/genes16020121. Genes (Basel). 2025. PMID: 40004449 Free PMC article.
-
Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health.Plants (Basel). 2025 Apr 19;14(8):1247. doi: 10.3390/plants14081247. Plants (Basel). 2025. PMID: 40284136 Free PMC article. Review.
-
A comprehensive analysis of the WRKY family in soybean and functional analysis of GmWRKY164-GmGSL7c in resistance to soybean mosaic virus.BMC Genomics. 2024 Jun 19;25(1):620. doi: 10.1186/s12864-024-10523-8. BMC Genomics. 2024. PMID: 38898399 Free PMC article.
-
Identification of maize genes that condition early systemic infection of sugarcane mosaic virus through single-cell transcriptomics.Plant Commun. 2025 May 12;6(5):101297. doi: 10.1016/j.xplc.2025.101297. Epub 2025 Mar 4. Plant Commun. 2025. PMID: 40045576 Free PMC article.
References
-
- Alazem M, Lin KY, Lin NS. 2014. The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Molecular Plant-Microbe Interactions: MPMI 27, 177–189. - PubMed
-
- Beckers GJ, Conrath U. 2007. Priming for stress resistance: from the lab to the field. Current Opinion in Plant Biology 10, 425–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

