Viral Involvement in Alzheimer's Disease
- PMID: 33687205
- PMCID: PMC8033564
- DOI: 10.1021/acschemneuro.0c00719
Viral Involvement in Alzheimer's Disease
Abstract
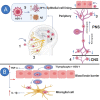
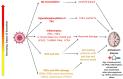
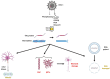
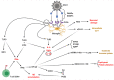
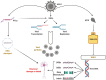
Alzheimer's disease (AD) is characterized by the presence of β-amyloid plaques (Aβ) and neurofibrillary tangles (NFTs) in the brain. The prevalence of the disease is increasing and is expected to reach 141 million cases by 2050. Despite the risk factors associated with the disease, there is no known causative agent for AD. Clinical trials with many drugs have failed over the years, and no therapeutic has been approved for AD. There is increasing evidence that pathogens are found in the brains of AD patients and controls, such as human herpes simplex virus-1 (HSV-1). Given the lack of a human model, the route for pathogen entry into the brain remains open for scrutiny and may include entry via a disturbed blood-brain barrier or the olfactory nasal route. Many factors can contribute to the pathogenicity of HSV-1, such as the ability of HSV-1 to remain latent, tau protein phosphorylation, increased accumulation of Aβ invivo and in vitro, and repeated cycle of reactivation if immunocompromised. Intriguingly, valacyclovir, a widely used drug for the treatment of HSV-1 and HSV-2 infection, has shown patient improvement in cognition compared to controls in AD clinical studies. We discuss the potential role of HSV-1 in AD pathogenesis and argue for further studies to investigate this relationship.
Keywords: Alzheimer’s disease; apolipoprotein E; blood−brain barrier; herpes simplex virus; valacyclovir; β-amyloid.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

