Targeting alveolar-specific succinate dehydrogenase A attenuates pulmonary inflammation during acute lung injury
- PMID: 33687752
- PMCID: PMC8250206
- DOI: 10.1096/fj.202002778R
Targeting alveolar-specific succinate dehydrogenase A attenuates pulmonary inflammation during acute lung injury
Abstract
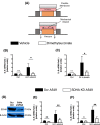
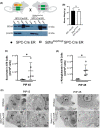
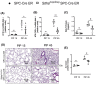
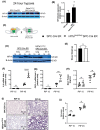
Acute lung injury (ALI) is an inflammatory lung disease, which manifests itself in patients as acute respiratory distress syndrome (ARDS). Previous studies have implicated alveolar-epithelial succinate in ALI protection. Therefore, we hypothesized that targeting alveolar succinate dehydrogenase SDH A would result in elevated succinate levels and concomitant lung protection. Wild-type (WT) mice or transgenic mice with targeted alveolar-epithelial Sdha or hypoxia-inducible transcription factor Hif1a deletion were exposed to ALI induced by mechanical ventilation. Succinate metabolism was assessed in alveolar-epithelial via mass spectrometry as well as redox measurements and evaluation of lung injury. In WT mice, ALI induced by mechanical ventilation decreased SDHA activity and increased succinate in alveolar-epithelial. In vitro, cell-permeable succinate decreased epithelial inflammation during stretch injury. Mice with inducible alveolar-epithelial Sdha deletion (Sdhaloxp/loxp SPC-CreER mice) revealed reduced lung inflammation, improved alveolar barrier function, and attenuated histologic injury. Consistent with a functional role of succinate to stabilize HIF, Sdhaloxp/loxp SPC-CreER experienced enhanced Hif1a levels during hypoxia or ALI. Conversely, Hif1aloxp/loxp SPC-CreER showed increased inflammation with ALI induced by mechanical ventilation. Finally, wild-type mice treated with intra-tracheal dimethlysuccinate were protected during ALI. These data suggest that targeting alveolar-epithelial SDHA dampens ALI via succinate-mediated stabilization of HIF1A. Translational extensions of our studies implicate succinate treatment in attenuating alveolar inflammation in patients suffering from ARDS.
Keywords: ALI; ARDS; HIF; SDHA; alveolar epithelium; inflammation; mechanical ventilation; succinate.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526‐2533. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685‐1693. - PubMed
-
- Villar J, Blanco J, Anon JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932‐1941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

