Fat Induces Glucose Metabolism in Nontransformed Liver Cells and Promotes Liver Tumorigenesis
- PMID: 33687947
- PMCID: PMC7611295
- DOI: 10.1158/0008-5472.CAN-20-1954
Fat Induces Glucose Metabolism in Nontransformed Liver Cells and Promotes Liver Tumorigenesis
Abstract
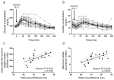
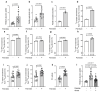
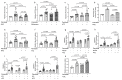
Hepatic fat accumulation is associated with diabetes and hepatocellular carcinoma (HCC). Here, we characterize the metabolic response that high-fat availability elicits in livers before disease development. After a short term on a high-fat diet (HFD), otherwise healthy mice showed elevated hepatic glucose uptake and increased glucose contribution to serine and pyruvate carboxylase activity compared with control diet (CD) mice. This glucose phenotype occurred independently from transcriptional or proteomic programming, which identifies increased peroxisomal and lipid metabolism pathways. HFD-fed mice exhibited increased lactate production when challenged with glucose. Consistently, administration of an oral glucose bolus to healthy individuals revealed a correlation between waist circumference and lactate secretion in a human cohort. In vitro, palmitate exposure stimulated production of reactive oxygen species and subsequent glucose uptake and lactate secretion in hepatocytes and liver cancer cells. Furthermore, HFD enhanced the formation of HCC compared with CD in mice exposed to a hepatic carcinogen. Regardless of the dietary background, all murine tumors showed similar alterations in glucose metabolism to those identified in fat exposed nontransformed mouse livers, however, particular lipid species were elevated in HFD tumor and nontumor-bearing HFD liver tissue. These findings suggest that fat can induce glucose-mediated metabolic changes in nontransformed liver cells similar to those found in HCC. SIGNIFICANCE: With obesity-induced hepatocellular carcinoma on a rising trend, this study shows in normal, nontransformed livers that fat induces glucose metabolism similar to an oncogenic transformation.
©2021 American Association for Cancer Research.
Conflict of interest statement
SMF has received funding from Bayer, Merck and Black Belt Therapeutics and has consulted for Fund+. All other authors declare to have no competing interest.
Figures




References
-
- Lundsgaard A-M, Fritzen AM, Kiens B. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends in Endocrinology & Metabolism. 2018;29(1):18–30. - PubMed
-
- Lorendeau D, Christen S, Rinaldi G, Fendt S-M. Metabolic control of signaling pathways and metabolic auto-regulation. Biology of the Cell. 2015;107(8):251–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

