Releasing the brakes of tumor immunity with anti-PD-L1 and pushing its accelerator with L19-IL2 cures poorly immunogenic tumors when combined with radiotherapy
- PMID: 33688020
- PMCID: PMC7944996
- DOI: 10.1136/jitc-2020-001764
Releasing the brakes of tumor immunity with anti-PD-L1 and pushing its accelerator with L19-IL2 cures poorly immunogenic tumors when combined with radiotherapy
Abstract
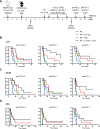
Background: Poorly immunogenic tumors are hardly responsive to immunotherapies such as immune checkpoint blockade (ICB) and are, therefore, a therapeutic challenge. Combination with other immunotherapies and/or immunogenic therapies, such as radiotherapy (RT), could make these tumors more immune responsive. We have previously shown that the immunocytokine L19-IL2 combined with single-dose RT resulted in 75% tumor remission and a 20% curative abscopal effect in the T cell-inflamed C51 colon carcinoma model. This treatment schedule was associated with the upregulation of inhibitory immune checkpoint (IC) molecules on tumor-infiltrating T cells, leading to only tumor growth delay in the poorly immunogenic Lewis lung carcinoma (LLC) model.
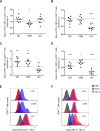
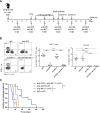
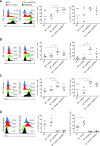
Methods: We aimed to trigger curative therapeutic responses in three tumor models (LLC, C51 and CT26) by "pushing the accelerator" of tumor immunity with L19-IL2 and/or "releasing the brakes" with ICB, such as antibodies directed against cytotoxic T lymphocyte associated protein 4 (CTLA-4), programmed death 1 (PD-1) or its ligand (PD-L1), combined with single-dose RT (10 Gy or 5 Gy). Primary tumor endpoint was defined as time to reach four times the size of tumor volume at start of treatment (4T×SV). Multivariate analysis of 4T×SV was performed using the Cox proportional hazards model comparing each treatment group with controls. Causal involvement of T and natural killer (NK) cells in the anti-tumor effect was assessed by in vivo depletion of T, NK or both cell populations. Immune profiling was performed using flow cytometry on single cell suspensions from spleens, bone marrow, tumors and blood.
Results: Combining RT, anti-PD-L1 and L19-IL2 cured 38% of LLC tumors, which was both CD8+ T and NK cell dependent. LLC tumors were resistant to RT +anti-PD-L1 likely explained by the upregulation of other IC molecules and increased T regulatory cell tumor infiltration. RT+L19-IL2 outperformed RT+ICB in C51 tumors; effects were comparable in CT26 tumors. Triple combinations were not superior to RT+L19-IL2 in both these models.
Conclusions: This study demonstrated that combinatorial strategies rationally designed on biological effects can turn immunotherapy-resistant tumors into immunologically responsive tumors. This hypothesis is currently being tested in the international multicentric randomized phase 2 trial: ImmunoSABR (NCT03705403).
Keywords: immunotherapy; radiotherapy; tumor microenvironment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: PL reports, within the submitted work, an in kind donation of Philogen (L19-IL2) and a status of PI of the trial ImmunoSABR. DN reports, within the submitted work, his role as co-founder and president of the Scientific Advisory Board of Philogen. PL reports outside the submitted work grants/sponsored research agreements from Varian medical, Oncoradiomics, ptTheragnostic/DNAmito and Health Innovation Ventures. He received an advisor/presenter fee and/or reimbursement of travel costs/external grant writing fee and/or in kind manpower contribution from Oncoradiomics, BHV, Merck, Varian, Elekta, ptTheragnostic and Convert pharmaceuticals. PL has shares in the company Oncoradiomics SA, Convert pharmaceuticals SA and The Medical Cloud Company SPRL and is co-inventor of two issued patents with royalties on radiomics (PCT/NL2014/050248 and PCT/NL2014/050728) licensed to Oncoradiomics and one issued patent on mtDNA (PCT/EP2014/059089) licensed to ptTheragnostic/DNAmito, three non-patented invention (software) licensed to ptTheragnostic/DNAmito, Oncoradiomics and Health Innovation Ventures and three non-issued, non-licensed patents on Deep Learning-Radiomics and Lymphocytes Sparing Radiotherapy. LJD is co-inventor of a non-issued, non-licensed patent on Lymphocyte Sparing Radiotherapy.
Figures



References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
