Circulating cytokines associated with clinical response to systemic therapy in metastatic renal cell carcinoma
- PMID: 33688021
- PMCID: PMC7944971
- DOI: 10.1136/jitc-2020-002009
Circulating cytokines associated with clinical response to systemic therapy in metastatic renal cell carcinoma
Abstract
Background: Circulating cytokines and angiogenic factors have been associated with clinical outcomes in patients with metastatic renal cell carcinoma (RCC) receiving systemic therapy. However, none have yet examined cytokine concentrations in parallel cohorts receiving either immunotherapy or targeted therapy.
Methods: In this prospective correlative study, we enrolled 56 patients who were planned for treatment with either a vascular endothelial growth factor-tyrosine kinase inhibitor (VEGF-TKI) or immune checkpoint inhibitor (ICI). Eligibility requirements permitted any RCC histologic subtype, International Metastatic Renal Cell Carcinoma risk classification, and line of therapy. Immunologic profile was assessed at baseline and after 1 month on treatment using a Human Cytokine 30-plex protein assay (Invitrogen). Clinical benefit was defined as complete response, partial response, or stable disease ≥6 months per RECIST (Response Evaluation Criteria in Solid Tumors) V.1.1 criteria.
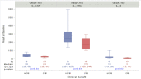
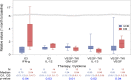
Results: Clinical benefit was similar between VEGF-TKI and ICI arms (65% vs 54%). Patients with clinical benefit from VEGF-TKIs had lower pretreatment levels of interleukin-6 (IL-6) (p=0.02), IL-1RA (p=0.03), and granulocyte colony-stimulating factor (CSF) (p=0.02). At 1 month, patients with clinical benefit from ICIs had higher levels of interferon-γ (IFN-γ) (p=0.04) and IL-12 (p=0.03). Among patients on VEGF-TKIs, those with clinical benefit had lower 1 month IL-13 (p=0.02) and granulocyte macrophage CSF (p=0.01) as well as higher 1 month VEGF (p=0.04) compared with patients with no clinical benefit.
Conclusion: For patients receiving VEGF-TKI or ICI therapy, distinct plasma cytokines were associated with clinical benefit. Our findings support additional investigation into plasma cytokines as biomarkers in metastatic RCC.
Keywords: biomarkers; cytokines; immunotherapy; tumor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SP reports consulting roles in Genentech, Aveo, Eisai, Roche, Pfizer, Novartis, Exelixis, Ipsen, BMS, and Astellas. ACR, LM, MA, ND, PB, NS, JH, NR, EK, and MK declare no conflicts of interest.
Figures
Similar articles
-
Circulating immune biomarkers correlating with response in patients with metastatic renal cell carcinoma on immunotherapy.JCI Insight. 2025 Jan 7;10(4):e185963. doi: 10.1172/jci.insight.185963. JCI Insight. 2025. PMID: 39773530 Free PMC article.
-
Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma.Eur J Cancer. 2020 Aug;135:203-210. doi: 10.1016/j.ejca.2020.05.009. Epub 2020 Jun 27. Eur J Cancer. 2020. PMID: 32599410
-
Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma.J Exp Clin Cancer Res. 2021 Jun 7;40(1):186. doi: 10.1186/s13046-021-01961-3. J Exp Clin Cancer Res. 2021. PMID: 34099013 Free PMC article. Review.
-
The Therapeutic Landscape of Renal Cell Carcinoma: From the Dark Age to the Golden Age.Semin Nephrol. 2020 Jan;40(1):28-41. doi: 10.1016/j.semnephrol.2019.12.004. Semin Nephrol. 2020. PMID: 32130964 Free PMC article. Review.
-
NLR Outperforms Low Hemoglobin and High Platelet Count as Predictive and Prognostic Biomarker in Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors.Clin Genitourin Cancer. 2024 Jun;22(3):102072. doi: 10.1016/j.clgc.2024.102072. Epub 2024 Mar 8. Clin Genitourin Cancer. 2024. PMID: 38615487
Cited by
-
Overcoming Acquired Drug Resistance to Cancer Therapies through Targeted STAT3 Inhibition.Int J Mol Sci. 2023 Mar 1;24(5):4722. doi: 10.3390/ijms24054722. Int J Mol Sci. 2023. PMID: 36902166 Free PMC article. Review.
-
A comprehensive investigation discovered the novel methyltransferase METTL24 as one presumably prognostic gene for kidney renal clear cell carcinoma potentially modulating tumor immune microenvironment.Front Immunol. 2022 Oct 14;13:926461. doi: 10.3389/fimmu.2022.926461. eCollection 2022. Front Immunol. 2022. PMID: 36311770 Free PMC article.
-
Circulating immune biomarkers correlating with response in patients with metastatic renal cell carcinoma on immunotherapy.JCI Insight. 2025 Jan 7;10(4):e185963. doi: 10.1172/jci.insight.185963. JCI Insight. 2025. PMID: 39773530 Free PMC article.
-
Prognostic role of circulating cytokines and inflammation indexes for avelumab maintenance in metastatic urothelial carcinoma.Front Immunol. 2024 May 10;15:1401214. doi: 10.3389/fimmu.2024.1401214. eCollection 2024. Front Immunol. 2024. PMID: 38799450 Free PMC article.
-
The tumor and plasma cytokine profiles of renal cell carcinoma patients.Sci Rep. 2022 Aug 4;12(1):13416. doi: 10.1038/s41598-022-17592-3. Sci Rep. 2022. PMID: 35927313 Free PMC article.
References
-
- National Cancer Institute . Drugs Approved for kidney (renal cell) cancer, 2011. Available: https://www.cancer.gov/about-cancer/treatment/drugs/kidney
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
