The Negative Allosteric Modulator EU1794-4 Reduces Single-Channel Conductance and Ca2+ Permeability of GluN1/GluN2A N-Methyl-d-Aspartate Receptors
- PMID: 33688039
- PMCID: PMC8058507
- DOI: 10.1124/molpharm.120.000218
The Negative Allosteric Modulator EU1794-4 Reduces Single-Channel Conductance and Ca2+ Permeability of GluN1/GluN2A N-Methyl-d-Aspartate Receptors
Abstract
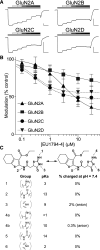
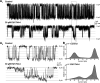
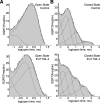
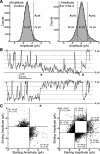
NMDA receptors are ligand-gated ion channels that mediate a slow, Ca2+-permeable component of excitatory synaptic currents. These receptors are involved in several important brain functions, including learning and memory, and have also been implicated in neuropathological conditions and acute central nervous system injury, which has driven therapeutic interest in their modulation. The EU1794 series of positive and negative allosteric modulators of NMDA receptors has structural determinants of action near the preM1 helix that is involved in channel gating. Here, we describe the effects of the negative allosteric modulator EU1794-4 on GluN1/GluN2A channels studied in excised outside-out patches. Coapplication of EU1794-4 with a maximally effective concentration of glutamate and glycine increases the fraction of time the channel is open by nearly 1.5-fold, yet reduces single-channel conductance by increasing access of the channel to several subconductance levels, which has the net overall effect of reducing the macroscopic current. The lack of voltage-dependence of negative modulation suggests this is unrelated to a channel block mechanism. As seen with other NMDA receptor modulators that reduce channel conductance, EU1794-4 also reduces the Ca2+ permeability relative to monovalent cations of GluN1/GluN2A receptors. We conclude that EU1794-4 is a prototype for a new class of NMDA receptor negative allosteric modulators that reduce both the overall current that flows after receptor activation and the flux of Ca2+ ion relative to monovalent cations. SIGNIFICANCE STATEMENT: NMDA receptors are implicated in many neurological conditions but are challenging to target given their ubiquitous expression. Several newly identified properties of the negative allosteric modulator EU1794-4, including reducing Ca2+ flux through NMDA receptors and attenuating channel conductance, explain why this modulator reduces but does not eliminate NMDA receptor function. A modulator with these properties could have therapeutic advantages for indications in which attenuation of NMDA receptor function is beneficial, such as neurodegenerative disease and acute injury.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the funding agency. S.F.T. is a PI on research grants from Allergan, Biogen, and Janssen to Emory University; is a paid consultant for Janssen; is a member of the SAB for Sage Therapeutics, Eumentis, the GRIN2B Foundation, and the CureGRIN Foundation; is cofounder of NeurOp Inc. and Agrithera Inc.; and has received licensing fees and royalties from Emory. D.C.L. is on the Board of Directors of NeurOp Inc. S.F.T., B.K., H.Y., and D.C.L. are coinventors on Emory University–owned intellectual property that includes allosteric modulators of glutamate receptor function. H.Y. is a PI on research grant from Sage Therapeutics to Emory University.
Figures



Similar articles
-
Evaluation of allosteric N-methyl-d-aspartate receptor modulation by GluN2A-selective antagonists using pharmacological equilibrium modeling.Mol Pharmacol. 2025 Jan;107(1):100004. doi: 10.1124/molpharm.124.000975. Epub 2024 Nov 22. Mol Pharmacol. 2025. PMID: 39919165
-
Mechanisms of Action Underlying Conductance-Modifying Positive Allosteric Modulators of the NMDA Receptor.Mol Pharmacol. 2024 Nov 18;106(6):334-353. doi: 10.1124/molpharm.124.001019. Mol Pharmacol. 2024. PMID: 39443157
-
Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator.Mol Pharmacol. 2014 Nov;86(5):548-60. doi: 10.1124/mol.114.094516. Epub 2014 Sep 9. Mol Pharmacol. 2014. PMID: 25205677 Free PMC article.
-
Investigation of the structural requirements for N-methyl-D-aspartate receptor positive and negative allosteric modulators based on 2-naphthoic acid.Eur J Med Chem. 2019 Feb 15;164:471-498. doi: 10.1016/j.ejmech.2018.12.054. Epub 2018 Dec 28. Eur J Med Chem. 2019. PMID: 30622023 Free PMC article. Review.
-
Development of GluN2A NMDA receptor positive allosteric modulators: Recent advances and perspectives.Bioorg Med Chem. 2025 Jul 1;124:118194. doi: 10.1016/j.bmc.2025.118194. Epub 2025 Apr 10. Bioorg Med Chem. 2025. PMID: 40239379 Review.
Cited by
-
Levodopa-induced dyskinesia: interplay between the N-methyl-D-aspartic acid receptor and neuroinflammation.Front Immunol. 2023 Oct 4;14:1253273. doi: 10.3389/fimmu.2023.1253273. eCollection 2023. Front Immunol. 2023. PMID: 37860013 Free PMC article. Review.
-
Development of a Dihydroquinoline-Pyrazoline GluN2C/2D-Selective Negative Allosteric Modulator of the N-Methyl-d-aspartate Receptor.ACS Chem Neurosci. 2023 Sep 6;14(17):3059-3076. doi: 10.1021/acschemneuro.3c00181. Epub 2023 Aug 11. ACS Chem Neurosci. 2023. PMID: 37566734 Free PMC article.
-
Activity-dependent diffusion trapping of AMPA receptors as a key step for expression of early LTP.Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230220. doi: 10.1098/rstb.2023.0220. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853553 Free PMC article. Review.
-
Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System.Int J Mol Sci. 2023 May 15;24(10):8803. doi: 10.3390/ijms24108803. Int J Mol Sci. 2023. PMID: 37240147 Free PMC article. Review.
-
Selective Reduction of Ca2+ Entry Through the Human NMDA Receptor: a Quantitative Study by Simultaneous Ca2+ and Na+ Imaging.Mol Neurobiol. 2024 Aug;61(8):5841-5850. doi: 10.1007/s12035-024-03944-9. Epub 2024 Jan 19. Mol Neurobiol. 2024. PMID: 38240993 Free PMC article.
References
-
- Albers GW, Goldstein LB, Hall D, Lesko LM, Aptiganel Acute Stroke Investigators (2001) Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA 286:2673–2682. - PubMed
-
- Attanasi OA, De Crescentini L, Favi G, Filippone P, Giorgi G, Mantellini F, Perrulli FR, Spinelli D (2008) Simple construction of fused and spiro nitrogen/sulfur containing heterocycles by addition of thioamides or thioureas on cycloalkenyl-diazenes: examples of click chemistry. Tetrahedron 64:3837–3858.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

