Ketamine-Induced Alteration of Working Memory Utility during Oculomotor Foraging Task in Monkeys
- PMID: 33688041
- PMCID: PMC8026253
- DOI: 10.1523/ENEURO.0403-20.2021
Ketamine-Induced Alteration of Working Memory Utility during Oculomotor Foraging Task in Monkeys
Abstract
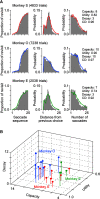
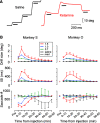
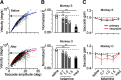
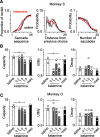
Impairments of working memory (WM) are commonly observed in a variety of neurodegenerative disorders but they are difficult to quantitatively assess in clinical cases. Recent studies in experimental animals have used low-dose ketamine (an NMDA receptor antagonist) to disrupt WM, partly mimicking the pathophysiology of schizophrenia. Here, we developed a novel behavioral paradigm to assess multiple components of WM and applied it to monkeys with and without ketamine administration. In an oculomotor foraging task, the animals were presented with 15 identical objects on the screen. One of the objects was associated with a liquid reward, and monkeys were trained to search for the target by generating sequential saccades under a time constraint. We assumed that the occurrence of recursive movements to the same object might reflect WM dysfunction. We constructed a "foraging model" that incorporated (1) memory capacity, (2) memory decay, and (3) utility rate; this model was able to explain more than 92% of the variations in behavioral data obtained from three monkeys. Following systemic administration of low dosages of ketamine, the memory capacity and utility rate were dramatically reduced by 15% and 57%, respectively, while memory decay remained largely unchanged. These results suggested that the behavioral deficits during the blockade of NMDA receptors were mostly due to the decreased usage of short-term memory. Our oculomotor paradigm and foraging model appear to be useful for quantifying multiple components of WM and could be applicable to clinical cases in future studies.
Keywords: NMDA receptor antagonist; central executive; exploratory behavior; nonhuman primate; recursive choice; visual search.
Copyright © 2021 Sawagashira and Tanaka.
Figures

References
-
- Aks DJ, Zelinsky GJ, Sprott JC (2002) Memory across eye-movements: 1/f dynamic in visual search. Nonlinear Dynamics Psychol Life Sci 6:1–25. 10.1023/A:1012222601935 - DOI
-
- Baddeley AD, Hitch G (1974) Working memory. Psychol Learn Motiv 8:47–89.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
