Photosynthesis tunes quantum-mechanical mixing of electronic and vibrational states to steer exciton energy transfer
- PMID: 33688046
- PMCID: PMC7980405
- DOI: 10.1073/pnas.2018240118
Photosynthesis tunes quantum-mechanical mixing of electronic and vibrational states to steer exciton energy transfer
Abstract
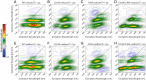
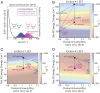
Photosynthetic species evolved to protect their light-harvesting apparatus from photoxidative damage driven by intracellular redox conditions or environmental conditions. The Fenna-Matthews-Olson (FMO) pigment-protein complex from green sulfur bacteria exhibits redox-dependent quenching behavior partially due to two internal cysteine residues. Here, we show evidence that a photosynthetic complex exploits the quantum mechanics of vibronic mixing to activate an oxidative photoprotective mechanism. We use two-dimensional electronic spectroscopy (2DES) to capture energy transfer dynamics in wild-type and cysteine-deficient FMO mutant proteins under both reducing and oxidizing conditions. Under reducing conditions, we find equal energy transfer through the exciton 4-1 and 4-2-1 pathways because the exciton 4-1 energy gap is vibronically coupled with a bacteriochlorophyll-a vibrational mode. Under oxidizing conditions, however, the resonance of the exciton 4-1 energy gap is detuned from the vibrational mode, causing excitons to preferentially steer through the indirect 4-2-1 pathway to increase the likelihood of exciton quenching. We use a Redfield model to show that the complex achieves this effect by tuning the site III energy via the redox state of its internal cysteine residues. This result shows how pigment-protein complexes exploit the quantum mechanics of vibronic coupling to steer energy transfer.
Keywords: excitonic energy transfer; photosynthesis; quantum effects in biology; ultrafast spectroscopy; vibronic coupling.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Fenna R. E., Matthews B. W., Chlorophyll arrangement in a bacteriochlorophyll protein from Chlorobium limicola. Nature 258, 573–577 (1975).
-
- Liu Z., et al., Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004). - PubMed
-
- Blankenship R. E., Molecular Mechanisms of Photosynthesis (Wiley/Blackwell, Chichester, ed. 2, 2014).
-
- Saer R., et al., Perturbation of bacteriochlorophyll molecules in Fenna-Matthews-Olson protein complexes through mutagenesis of cysteine residues. Biochim. Biophys. Acta 1857, 1455–1463 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

