The diversity and evolution of microbial dissimilatory phosphite oxidation
- PMID: 33688048
- PMCID: PMC7980464
- DOI: 10.1073/pnas.2020024118
The diversity and evolution of microbial dissimilatory phosphite oxidation
Abstract
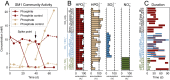
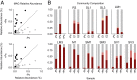
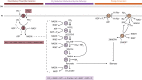
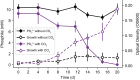
Phosphite is the most energetically favorable chemotrophic electron donor known, with a half-cell potential (Eo') of -650 mV for the PO43-/PO33- couple. Since the discovery of microbial dissimilatory phosphite oxidation (DPO) in 2000, the environmental distribution, evolution, and diversity of DPO microorganisms (DPOMs) have remained enigmatic, as only two species have been identified. Here, metagenomic sequencing of phosphite-enriched microbial communities enabled the genome reconstruction and metabolic characterization of 21 additional DPOMs. These DPOMs spanned six classes of bacteria, including the Negativicutes, Desulfotomaculia, Synergistia, Syntrophia, Desulfobacteria, and Desulfomonilia_A Comparing the DPO genes from the genomes of enriched organisms with over 17,000 publicly available metagenomes revealed the global existence of this metabolism in diverse anoxic environments, including wastewaters, sediments, and subsurface aquifers. Despite their newfound environmental and taxonomic diversity, metagenomic analyses suggested that the typical DPOM is a chemolithoautotroph that occupies low-oxygen environments and specializes in phosphite oxidation coupled to CO2 reduction. Phylogenetic analyses indicated that the DPO genes form a highly conserved cluster that likely has ancient origins predating the split of monoderm and diderm bacteria. By coupling microbial cultivation strategies with metagenomics, these studies highlighted the unsampled metabolic versatility latent in microbial communities. We have uncovered the unexpected prevalence, diversity, biochemical specialization, and ancient origins of a unique metabolism central to the redox cycling of phosphorus, a primary nutrient on Earth.
Keywords: CO2 fixation; Desulfotignum; Phosphitivorax; glycine reductive pathway; phosphite.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Figueroa I. A., Coates J. D., Microbial phosphite oxidation and its potential role in the global phosphorus and carbon cycles. Adv. Appl. Microbiol. 98, 93–117 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

