An early global role for Axin is required for correct patterning of the anterior-posterior axis in the sea urchin embryo
- PMID: 33688076
- PMCID: PMC8034878
- DOI: 10.1242/dev.191197
An early global role for Axin is required for correct patterning of the anterior-posterior axis in the sea urchin embryo
Abstract
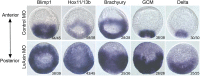
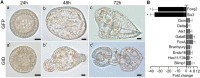
Activation of Wnt/β-catenin (cWnt) signaling at the future posterior end of early bilaterian embryos is a highly conserved mechanism for establishing the anterior-posterior (AP) axis. Moreover, inhibition of cWnt at the anterior end is required for development of anterior structures in many deuterostome taxa. This phenomenon, which occurs around the time of gastrulation, has been fairly well characterized, but the significance of intracellular inhibition of cWnt signaling in cleavage-stage deuterostome embryos for normal AP patterning is less well understood. To investigate this process in an invertebrate deuterostome, we defined Axin function in early sea urchin embryos. Axin is ubiquitously expressed at relatively high levels in early embryos and functional analysis revealed that Axin suppresses posterior cell fates in anterior blastomeres by blocking ectopic cWnt activation in these cells. Structure-function analysis of sea urchin Axin demonstrated that only its GSK-3β-binding domain is required for cWnt inhibition. These observations and results in other deuterostomes suggest that Axin plays a crucial conserved role in embryonic AP patterning by preventing cWnt activation in multipotent early blastomeres, thus protecting them from assuming ectopic cell fates.
Keywords: Animal-vegetal axis; Anterior-posterior axis; Axin; Endomesoderm; Sea urchin; Wnt signaling.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests. Author contributionsConceptualization: H.S., C.J.P., A.H.W.; Methodology: H.S., C.J.P., H.F., A.H.W.; Validation: H.S., C.J.P., L.W., A.H.W.; Formal analysis: H.S., C.J.P., L.W., H.F., A.H.W.; Investigation: H.S., C.J.P., L.W., A.H.W.; Resources: A.H.W.; Data curation: H.S., C.J.P., L.W., H.F., A.H.W.; Writing - original draft: H.S., C.J.P., A.H.W.; Writing - review & editing: H.S., A.H.W.; Visualization: H.S.; Supervision: A.H.W.; Project administration: A.H.W.; Funding acquisition: A.H.W.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

