Estimation of treatment and prognostic factors of pneumocystis pneumonia in patients with connective tissue diseases
- PMID: 33688083
- PMCID: PMC7944977
- DOI: 10.1136/rmdopen-2020-001508
Estimation of treatment and prognostic factors of pneumocystis pneumonia in patients with connective tissue diseases
Abstract
Objectives: To investigate short-term prognosis and prognostic factors for connective tissue disease-associated pneumocystis pneumonia (CTD-PCP) using the Japanese nationwide diagnosis procedure combination (DPC) inpatient database.
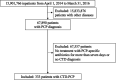
Methods: The present retrospective cohort study from April 2014 to March 2016 included data of patients with CTD-PCP extracted from the DPC database using the 10th revision of International Classification of Diseases and Injuries codes.
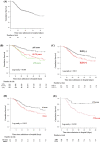
Results: In 15 901 766 cases registered from 1329 hospitals, 333 of 67 890 patients who were admitted with PCP were diagnosed with CTD-PCP and included in the study. The median age was 71.0 years, and 214 (64.3%), 80 (24.0%), and 29 (8.7%) patients received sulfamethoxazole/trimethoprim (ST) monotherapy and pentamidine-containing and atovaquone-containing therapy, respectively. There were 114 (34.2%) in-hospital deaths, and the 30-day and 60-day in-hospital survival rates after PCP treatment initiation were 66.0% and 53.7%, respectively. Older age (HR 1.06, 95% CI 1.03 to 1.08) and concomitant interstitial lung disease (ILD) (HR 1.65, 95% CI 1.12 to 2.42) were poor prognostic factors. Patients who completed PCP treatment with ST monotherapy had a significantly higher survival rate than those treated with those not treated with ST monotherapy (p=0.015; log-rank test). Pentamidine versus atovaquone as second-line therapy was significantly higher with atovaquone (p=0.012; log-rank test).
Conclusion: Older age and concomitant ILD were poor prognostic factors for CTD-PCP. ST was a reasonable first-line therapy in patients with CTD-PCP, and patients with inadequate response to ST treated with atovaquone tended to have a better prognosis than those treated with pentamidine.
Keywords: autoimmune diseases; health care; inflammation; outcome assessment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YT has received speaking fees and/or honoraria from Daiichi-Sankyo, Astellas, Chugai, Eli Lilly, Pfizer, Abbvie, YL Biologics, Bristol-Myers, Takeda, Mitsubishi-Tanabe, Novartis, Eisai, Janssen, and Teijin and has received research grants from Asahi-kasei, Mitsubishi-Tanabe, Chugai, Takeda, Sanofi, Bristol-Myers, UCB, Daiichi-Sankyo, Eisai, and Ono. KN has received research grants from Mitsubishi-Tanabe, Eli Lilly, and Eisai.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
