The Value of a Seven-Autoantibody Panel Combined with the Mayo Model in the Differential Diagnosis of Pulmonary Nodules
- PMID: 33688380
- PMCID: PMC7914080
- DOI: 10.1155/2021/6677823
The Value of a Seven-Autoantibody Panel Combined with the Mayo Model in the Differential Diagnosis of Pulmonary Nodules
Abstract
Background: Identifying malignant pulmonary nodules and detecting early-stage lung cancer (LC) could reduce mortality. This study investigated the clinical value of a seven-autoantibody (7-AAB) panel in combination with the Mayo model for the early detection of LC and distinguishing benign from malignant pulmonary nodules (MPNs).
Methods: The concentrations of the elements of a 7-AAB panel were quantitated by enzyme-linked immunosorbent assay (ELISA) in 806 participants. The probability of MPNs was calculated using the Mayo predictive model. The performances of the 7-AAB panel and the Mayo model were analyzed by receiver operating characteristic (ROC) analyses, and the difference between groups was evaluated by chi-square tests (χ 2).
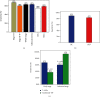
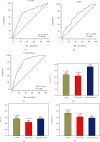
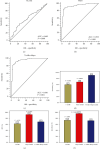
Results: The combined area under the ROC curve (AUC) for all 7 AABs was higher than that of a single one. The sensitivities of the 7-AAB panel were 67.5% in the stage I-II LC patients and 60.3% in the stage III-IV patients, with a specificity of 89.6% for the healthy controls and 83.1% for benign lung disease patients. The detection rate of the 7-AAB panel in the early-stage LC patients was higher than that of traditional tumor markers. The AUC of the 7-AAB panel in combination with the Mayo model was higher than that of the 7-AAB panel alone or the Mayo model alone in distinguishing MPN from benign nodules. For early-stage MPN, the sensitivity and specificity of the combination were 93.5% and 58.0%, respectively. For advanced-stage MPN, the sensitivity and specificity of the combination were 91.4% and 72.8%, respectively. The combination of the 7-AAB panel with the Mayo model significantly improved the detection rate of MPN, but the positive predictive value (PPV) and the specificity were not improved when compared with either the 7-AAB panel alone or the Mayo model alone.
Conclusion: Our study confirmed the clinical value of the 7-AAB panel for the early detection of lung cancer and in combination with the Mayo model could be used to distinguish benign from malignant pulmonary nodules.
Copyright © 2021 Zhougui Ling et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Comparative Study of Autoantibody Responses between Lung Adenocarcinoma and Benign Pulmonary Nodules.J Thorac Oncol. 2016 Mar;11(3):334-45. doi: 10.1016/j.jtho.2015.11.011. Epub 2016 Feb 16. J Thorac Oncol. 2016. PMID: 26896032
-
[Value of Combined Detection of Cytokines and Tumor Markers in the Differential Diagnosis of Benign and Malignant Solitary Pulmonary Nodules].Zhongguo Fei Ai Za Zhi. 2021 Jun 20;24(6):426-433. doi: 10.3779/j.issn.1009-3419.2021.102.20. Zhongguo Fei Ai Za Zhi. 2021. PMID: 34157802 Free PMC article. Chinese.
-
The diagnostic value of a seven-autoantibody panel and a nomogram with a scoring table for predicting the risk of non-small-cell lung cancer.Cancer Sci. 2020 May;111(5):1699-1710. doi: 10.1111/cas.14371. Epub 2020 Mar 19. Cancer Sci. 2020. PMID: 32108977 Free PMC article.
-
Pulmonary nodule malignancy probability: a diagnostic accuracy meta-analysis of the Mayo model.Clin Radiol. 2022 Jun;77(6):443-450. doi: 10.1016/j.crad.2022.01.055. Epub 2022 Apr 11. Clin Radiol. 2022. PMID: 35422255
-
Autoantibodies as diagnostic biomarkers for lung cancer: A systematic review.Cell Death Discov. 2019 Aug 5;5:126. doi: 10.1038/s41420-019-0207-1. eCollection 2019. Cell Death Discov. 2019. PMID: 31396403 Free PMC article. Review.
Cited by
-
Rare giant ovarian metastasis arising from a small primary lung adenocarcinoma: a case report.Front Surg. 2023 Sep 12;10:1278076. doi: 10.3389/fsurg.2023.1278076. eCollection 2023. Front Surg. 2023. PMID: 37780910 Free PMC article. Review.
-
AI-enhanced diagnostic model for pulmonary nodule classification.Front Oncol. 2024 Aug 30;14:1417753. doi: 10.3389/fonc.2024.1417753. eCollection 2024. Front Oncol. 2024. PMID: 39281372 Free PMC article.
-
Validation of a High-Specificity Blood Autoantibody Test to Detect Lung Cancer in Pulmonary Nodules.CHEST Pulm. 2025 Mar;3(1):100130. doi: 10.1016/j.chpulm.2024.100130. Epub 2024 Dec 25. CHEST Pulm. 2025. PMID: 40296864 Free PMC article.
-
Precise diagnosis and prognosis assessment of malignant lung nodules: a narrative review.J Thorac Dis. 2024 Nov 30;16(11):7999-8013. doi: 10.21037/jtd-24-1058. Epub 2024 Nov 29. J Thorac Dis. 2024. PMID: 39678870 Free PMC article. Review.
-
Clinical application of serum seven tumour-associated autoantibodies in patients with pulmonary nodules.Heliyon. 2024 May 3;10(9):e30576. doi: 10.1016/j.heliyon.2024.e30576. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765082 Free PMC article.
References
-
- Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2019. CA: a Cancer Journal for Clinicians. 2018;69:7–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

