Review of investigational drugs for coronavirus disease 2019
- PMID: 33688540
- PMCID: PMC7933614
- DOI: 10.4103/jehp.jehp_457_20
Review of investigational drugs for coronavirus disease 2019
Abstract
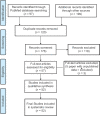
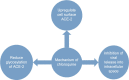
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became evident in Wuhan, China, and then spread rapidly worldwide. Numerous drugs and vaccines are under clinical trial pipeline for investigation against coronavirus disease 2019 (COVID-19) infection. The aim of this systematic review was to discuss about investigational new as well as repurposed drugs currently under trial for COVID-19 infection. An exhaustive search was carried out for this review article including scientific databases of PubMed, Embase, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, Web of Science, ScienceDirect, ProQuest, Google Scholar, and Scopus search engines using keywords of "Coronavirus," "COVID-19," "MERS-CoV," "MERS," "SARS-CoV-2," and "SARS-CoV-1" and "Solidarity trial" and their Persian-equivalent keywords from inception until May 2020. After screening the 296 articles searched from different databases (PubMed = 97 and other search engines = 199), 52 articles were included in the final systematic review. It was found that the World Health Organization introduced a Solidarity international clinical trial to discover an effectual treatment of COVID-19. Based on established in vitro and in vivo activity against different strains of coronaviruses, four repurposed drugs - remdesivir, lopinavir/ ritonavir combination, lopinavir/ritonavir with beta-1a, chloroquine, and hydroxychloroquine - were considered for clinical trial against COVID-19. A number of other drugs and vaccines are under clinical trial pipeline for investigation against COVID-19 infection. Despite multitude of treatment options available, treatment of choice is still not well established. Moreover, optimum supportive care and monitoring of seriously ill patients is the need of the hour.
Keywords: Coronavirus; Solidarity trial; coronavirus disease 2019; severe acute respiratory syndrome coronavirus 2.
Copyright: © 2021 Journal of Education and Health Promotion.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. - PubMed
-
- World Health Organization. SARS (Severe Acute Respiratory Syndrome) World Health Organization; [Last accessed on 2020 Apr 27]. Available from: https://www.who.int/ith/diseases/sars/en/
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous