Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle
- PMID: 33688609
- PMCID: PMC7935041
- DOI: 10.1162/netn_a_00169
Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle
Abstract
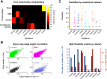
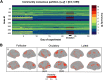
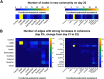
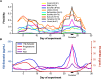
Sex steroid hormones have been shown to alter regional brain activity, but the extent to which they modulate connectivity within and between large-scale functional brain networks over time has yet to be characterized. Here, we applied dynamic community detection techniques to data from a highly sampled female with 30 consecutive days of brain imaging and venipuncture measurements to characterize changes in resting-state community structure across the menstrual cycle. Four stable functional communities were identified, consisting of nodes from visual, default mode, frontal control, and somatomotor networks. Limbic, subcortical, and attention networks exhibited higher than expected levels of nodal flexibility, a hallmark of between-network integration and transient functional reorganization. The most striking reorganization occurred in a default mode subnetwork localized to regions of the prefrontal cortex, coincident with peaks in serum levels of estradiol, luteinizing hormone, and follicle stimulating hormone. Nodes from these regions exhibited strong intranetwork increases in functional connectivity, leading to a split in the stable default mode core community and the transient formation of a new functional community. Probing the spatiotemporal basis of human brain-hormone interactions with dynamic community detection suggests that hormonal changes during the menstrual cycle result in temporary, localized patterns of brain network reorganization.
Keywords: Dense sampling; Dynamic community detection; Network flexibility; Sex hormones.
© 2020 Massachusetts Institute of Technology.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures


References
-
- Arélin, K., Mueller, K., Barth, C., Rekkas, P., Kratzsch, J., Burmann, I., … Sacher, J. (2015). Progesterone mediates brain functional connectivity changes during the menstrual cycle—A pilot resting state MRI study. Frontiers in Neuroscience, 9(44). DOI:https://doi.org/10.3389/fnins.2015.00044, PMID:25755630, PMCID:PMC4337344 - DOI - PMC - PubMed
-
- Bassett, D., Porter, M., Wymbs, N., Grafton, S., Carlson, J., & Mucha, P. (2013). Robust detection of dynamic community structure in networks. Chaos, 23, 01342. DOI:https://doi.org/10.1063/1.4790830, PMID:23556979, PMCID:PMC3618100 - DOI - PMC - PubMed
-
- Bassett, D., & Sporns, O. (2017). Network neuroscience. Nature Neuroscience, 20(3), 353–364. DOI:https://doi.org/10.1038/nn.4502, PMID:28230844, PMCID:PMC5485642 - DOI - PMC - PubMed
-
- Bassett, D., Wymbs, N., Porter, M., Mucha, P., Carlson, J., & Grafton, S. (2011). Dynamic reconfiguration of human brain networks during learning. PNAS, 108(18), 7641–7646. DOI:https://doi.org/10.1073/pnas.1018985108, PMID:21502525, PMCID:PMC3088578 - DOI - PMC - PubMed
-
- Bell, P., & Shine, J. (2016). Subcortical contributions to large-scale network communication. Neuroscience and Biobehavioral Reviews, 71, 313–322. DOI:https://doi.org/10.1016/j.neubiorev.2016.08.036, PMID:27590830 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
