This is a preprint.
The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory
- PMID: 33688649
- PMCID: PMC7941620
- DOI: 10.1101/2021.03.04.430128
The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory
Update in
-
The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory.iScience. 2021 Dec 17;24(12):103479. doi: 10.1016/j.isci.2021.103479. Epub 2021 Nov 20. iScience. 2021. PMID: 34841223 Free PMC article.
Abstract
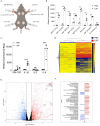
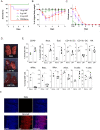
Vaccines based on mRNA-containing lipid nanoparticles (LNPs) are a promising new platform used by two leading vaccines against coronavirus disease in 2019 (COVID-19). Clinical trials and ongoing vaccinations present with very high protection levels and varying degrees of side effects. However, the nature of the reported side effects remains poorly defined. Here we present evidence that LNPs used in many preclinical studies are highly inflammatory in mice. Intradermal injection of these LNPs led to rapid and robust inflammatory responses, characterized by massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines. The same dose of LNP delivered intranasally led to similar inflammatory responses in the lung and resulted in a high mortality rate. In summary, here we show that the LNPs used for many preclinical studies are highly inflammatory. Thus, their potent adjuvant activity and reported superiority comparing to other adjuvants in supporting the induction of adaptive immune responses likely stem from their inflammatory nature. Furthermore, the preclinical LNPs are similar to the ones used for human vaccines, which could also explain the observed side effects in humans using this platform.
Keywords: LNP; SARS-CoV-2 vaccine; inflammation; mRNA-LNP vaccine; side-effects.
Conflict of interest statement
CONFLICT OF INTEREST Authors declare no conflict of any sort.
Figures


References
-
- Alameh M.-G., Weissman D., and Pardi N. (2020). Messenger RNA-Based Vaccines Against Infectious Diseases. p. - PubMed
-
- Bernasconi V., Norling K., Gribonika I., Ong L.C., Burazerovic S., Parveen N., Schön K., Stensson A., Bally M., Larson G., et al. (2021). A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 14, 523–536. - PubMed
-
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. (2020). SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
