This is a preprint.
Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity
- PMID: 33688673
- PMCID: PMC7941650
- DOI: 10.1101/2021.02.27.21252099
Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity
Update in
-
Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: A consecutive case series.EBioMedicine. 2021 May;67:103355. doi: 10.1016/j.ebiom.2021.103355. Epub 2021 Apr 26. EBioMedicine. 2021. PMID: 33915337 Free PMC article.
Abstract
Background: There is increasing concern that persistent infection of SARS-CoV-2 within immunocompromised hosts could serve as a reservoir for mutation accumulation and subsequent emergence of novel strains with the potential to evade immune responses.
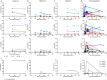
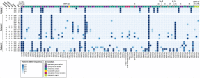
Methods: We describe three patients with acute lymphoblastic leukemia who were persistently positive for SARS-CoV-2 by real-time polymerase chain reaction. Viral viability from longitudinally-collected specimens was assessed. Whole-genome sequencing and serological studies were performed to measure viral evolution and evidence of immune escape.
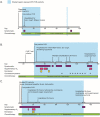
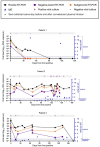
Findings: We found compelling evidence of ongoing replication and infectivity for up to 162 days from initial positive by subgenomic RNA, single-stranded RNA, and viral culture analysis. Our results reveal a broad spectrum of infectivity, host immune responses, and accumulation of mutations, some with the potential for immune escape.
Interpretation: Our results highlight the need to reassess infection control precautions in the management and care of immunocompromised patients. Routine surveillance of mutations and evaluation of their potential impact on viral transmission and immune escape should be considered.
Funding: The work was partially funded by The Saban Research Institute at Children's Hospital Los Angeles intramural support for COVID-19 Directed Research (X.G. and J.D.B.), the Johns Hopkins Center of Excellence in Influenza Research and Surveillance HHSN272201400007C (A.P.), NIH/NIAID R01AI127877 (S.D.B.), NIH/NIAID R01AI130398 (S.D.B.), NIH 1U54CA260517 (S.D.B.), an endowment to S.D.B. from the Crown Family Foundation, an Early Postdoc.Mobility Fellowship Stipend to O.F.W. from the Swiss National Science Foundation (SNSF), and a Coulter COVID-19 Rapid Response Award to S.D.B. L.G. is a SHARE Research Fellow in Pediatric Hematology-Oncology.
Conflict of interest statement
Conflict of Interest S.D.B. has consulted for Regeneron, Sanofi, and Novartis on topics unrelated to this study. S.D.B., and K.R. have filed provisional patent applications related to serological tests for SARS-CoV-2 antibodies. All other authors have no competing interests.
Figures
References
-
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020; 323: 2249. - PubMed
-
- CDC. COVID-19 and Your Health. Centers for Disease Control and Prevention. 2020; published online Feb 11. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html (accessed Dec 27, 2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
