Organ-on-chip of the cervical epithelial layer: A platform to study normal and pathological cellular remodeling of the cervix
- PMID: 33689188
- PMCID: PMC8193817
- DOI: 10.1096/fj.202002590RRR
Organ-on-chip of the cervical epithelial layer: A platform to study normal and pathological cellular remodeling of the cervix
Abstract
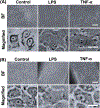
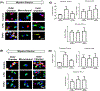
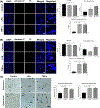
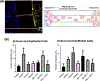
Damage to the cervical epithelial layer due to infection and inflammation is associated with preterm birth. However, the individual and/or collective roles of cervical epithelial layers in maintaining cervical integrity remain unclear during infection/inflammation. To determine the intercellular interactions, we developed an organ-on-chip of the cervical epithelial layer (CE-OOC) composed of two co-culture chambers connected by microchannels, recapitulating the ectocervical and endocervical epithelial layers. Further, we tested the interactions between cells from each distinct region and their contributions in maintaining cervical integrity in response to LPS and TNFα stimulations. The co-culture of ectocervical and endocervical cells facilitated cellular migration of both epithelial cells inside the microchannels. Compared to untreated controls, both LPS and TNFα increased apoptosis, necrosis, and senescence as well as increased pro-inflammatory cytokine productions by cervical epithelial cells. In summary, the CE-OOC established an in vitro model that can recapitulate the ectocervical and the endocervical epithelial regions of the cervix. The established CE-OOC may become a powerful tool in obstetrics and gynecology research such as in studying cervical remodeling during pregnancy and parturition and the dynamics of cervical epithelial cells in benign and malignant pathology in the cervix.
Keywords: cervical ripening; cervix; epithelial-to-mesenchymal transition; organ-on-a-chip; pregnancy; preterm birth.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflicts of interest regarding this study
Figures


Similar articles
-
Exosomes from Ureaplasma parvum-infected ectocervical epithelial cells promote feto-maternal interface inflammation but are insufficient to cause preterm delivery.Front Cell Dev Biol. 2022 Aug 15;10:931609. doi: 10.3389/fcell.2022.931609. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36046342 Free PMC article.
-
Progesterone alters human cervical epithelial and stromal cell transition and migration: Implications in cervical remodeling during pregnancy and parturition.Mol Cell Endocrinol. 2021 Jun 1;529:111276. doi: 10.1016/j.mce.2021.111276. Epub 2021 Apr 3. Mol Cell Endocrinol. 2021. PMID: 33823217 Free PMC article.
-
Modeling ascending Ureaplasma parvum infection through the female reproductive tract using vagina-cervix-decidua-organ-on-a-chip and feto-maternal interface-organ-on-a-chip.FASEB J. 2022 Oct;36(10):e22551. doi: 10.1096/fj.202200872R. FASEB J. 2022. PMID: 36106554 Free PMC article.
-
Cervical function in pregnancy and disease: new insights from single-cell analysis.Am J Obstet Gynecol. 2025 Apr;232(4S):S81-S94. doi: 10.1016/j.ajog.2024.07.039. Am J Obstet Gynecol. 2025. PMID: 40253084 Review.
-
Patient-derived and mouse endo-ectocervical organoid generation, genetic manipulation and applications to model infection.Nat Protoc. 2022 Jul;17(7):1658-1690. doi: 10.1038/s41596-022-00695-6. Epub 2022 May 11. Nat Protoc. 2022. PMID: 35546639 Review.
Cited by
-
Three-dimensional models of the cervicovaginal epithelia to study host-microbiome interactions and sexually transmitted infections.Pathog Dis. 2022 Aug 27;80(1):ftac026. doi: 10.1093/femspd/ftac026. Pathog Dis. 2022. PMID: 35927516 Free PMC article.
-
Inflammatory Amplification: A Central Tenet of Uterine Transition for Labor.Front Cell Infect Microbiol. 2021 Aug 19;11:660983. doi: 10.3389/fcimb.2021.660983. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34490133 Free PMC article. Review.
-
Exosomes from Ureaplasma parvum-infected ectocervical epithelial cells promote feto-maternal interface inflammation but are insufficient to cause preterm delivery.Front Cell Dev Biol. 2022 Aug 15;10:931609. doi: 10.3389/fcell.2022.931609. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36046342 Free PMC article.
-
Monocytes and cervical ripening: a narrative review of prolonged labor pathophysiology.Ann Med Surg (Lond). 2025 May 21;87(6):3289-3299. doi: 10.1097/MS9.0000000000003004. eCollection 2025 Jun. Ann Med Surg (Lond). 2025. PMID: 40486626 Free PMC article. Review.
-
Bioengineering trends in female reproduction: a systematic review.Hum Reprod Update. 2022 Nov 2;28(6):798-837. doi: 10.1093/humupd/dmac025. Hum Reprod Update. 2022. PMID: 35652272 Free PMC article.
References
-
- Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429–438. - PubMed
-
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

