Violacein-Induced Chaperone System Collapse Underlies Multistage Antiplasmodial Activity
- PMID: 33689276
- PMCID: PMC8042658
- DOI: 10.1021/acsinfecdis.0c00454
Violacein-Induced Chaperone System Collapse Underlies Multistage Antiplasmodial Activity
Abstract
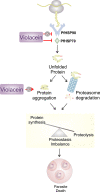
Antimalarial drugs with novel modes of action and wide therapeutic potential are needed to pave the way for malaria eradication. Violacein is a natural compound known for its biological activity against cancer cells and several pathogens, including the malaria parasite, Plasmodium falciparum (Pf). Herein, using chemical genomic profiling (CGP), we found that violacein affects protein homeostasis. Mechanistically, violacein binds Pf chaperones, PfHsp90 and PfHsp70-1, compromising the latter's ATPase and chaperone activities. Additionally, violacein-treated parasites exhibited increased protein unfolding and proteasomal degradation. The uncoupling of the parasite stress response reflects the multistage growth inhibitory effect promoted by violacein. Despite evidence of proteotoxic stress, violacein did not inhibit global protein synthesis via UPR activation-a process that is highly dependent on chaperones, in agreement with the notion of a violacein-induced proteostasis collapse. Our data highlight the importance of a functioning chaperone-proteasome system for parasite development and differentiation. Thus, a violacein-like small molecule might provide a good scaffold for development of a novel probe for examining the molecular chaperone network and/or antiplasmodial drug design.
Keywords: chaperone inhibitor; chemogenomics; malaria; proteostasis; violacein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
(-)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners.Molecules. 2017 Dec 5;22(12):2139. doi: 10.3390/molecules22122139. Molecules. 2017. PMID: 29206141 Free PMC article.
-
Extracts Obtained from Pterocarpus angolensis DC and Ziziphus mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function.Molecules. 2017 Jul 28;22(8):1224. doi: 10.3390/molecules22081224. Molecules. 2017. PMID: 28788073 Free PMC article.
-
Selective targeting of Plasmodium falciparum Hsp90 disrupts the 26S proteasome.Cell Chem Biol. 2024 Apr 18;31(4):729-742.e13. doi: 10.1016/j.chembiol.2024.02.008. Epub 2024 Mar 15. Cell Chem Biol. 2024. PMID: 38492573 Free PMC article.
-
Plasmodial Hsp40s: New Avenues for Antimalarial Drug Discovery.Curr Pharm Des. 2017 Nov 28;23(30):4555-4570. doi: 10.2174/1381612823666170124142439. Curr Pharm Des. 2017. PMID: 28120726 Review.
-
Co-chaperones of Hsp90 in Plasmodium falciparum and their concerted roles in cellular regulation.Parasitology. 2014 Aug;141(9):1177-91. doi: 10.1017/S0031182013002084. Epub 2014 Feb 21. Parasitology. 2014. PMID: 24560171 Review.
Cited by
-
Ursolic acid acetate and iso-mukaadial acetate bind to Plasmodium falciparum Hsp90, abrogating its chaperone function in vitro.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):5179-5192. doi: 10.1007/s00210-024-02944-9. Epub 2024 Jan 22. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38252299 Free PMC article.
-
Evaluation of Violacein Metabolic Stability and Metabolite Identification in Human, Mouse, and Rat Liver Microsomes.Pharmaceutics. 2025 May 2;17(5):601. doi: 10.3390/pharmaceutics17050601. Pharmaceutics. 2025. PMID: 40430892 Free PMC article.
-
Inhibitors of the Plasmodium falciparum Hsp90 towards Selective Antimalarial Drug Design: The Past, Present and Future.Cells. 2021 Oct 22;10(11):2849. doi: 10.3390/cells10112849. Cells. 2021. PMID: 34831072 Free PMC article. Review.
-
HSP70 and their co-chaperones in the human malaria parasite P. falciparum and their potential as drug targets.Front Mol Biosci. 2022 Aug 5;9:968248. doi: 10.3389/fmolb.2022.968248. eCollection 2022. Front Mol Biosci. 2022. PMID: 35992276 Free PMC article. Review.
-
Contemporary exploitation of natural products for arthropod-borne pathogen transmission-blocking interventions.Parasit Vectors. 2022 Aug 24;15(1):298. doi: 10.1186/s13071-022-05367-8. Parasit Vectors. 2022. PMID: 36002857 Free PMC article. Review.
References
-
- World Health Organization . World Malaria Report 2019; Geneva, 2019.
-
- Malaria: Strategy Overview; Bill Melinda Gates Foundation, 2011; April, pp 1–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

