Violacein-Induced Chaperone System Collapse Underlies Multistage Antiplasmodial Activity
- PMID: 33689276
- PMCID: PMC8042658
- DOI: 10.1021/acsinfecdis.0c00454
Violacein-Induced Chaperone System Collapse Underlies Multistage Antiplasmodial Activity
Abstract
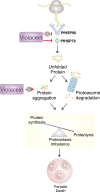
Antimalarial drugs with novel modes of action and wide therapeutic potential are needed to pave the way for malaria eradication. Violacein is a natural compound known for its biological activity against cancer cells and several pathogens, including the malaria parasite, Plasmodium falciparum (Pf). Herein, using chemical genomic profiling (CGP), we found that violacein affects protein homeostasis. Mechanistically, violacein binds Pf chaperones, PfHsp90 and PfHsp70-1, compromising the latter's ATPase and chaperone activities. Additionally, violacein-treated parasites exhibited increased protein unfolding and proteasomal degradation. The uncoupling of the parasite stress response reflects the multistage growth inhibitory effect promoted by violacein. Despite evidence of proteotoxic stress, violacein did not inhibit global protein synthesis via UPR activation-a process that is highly dependent on chaperones, in agreement with the notion of a violacein-induced proteostasis collapse. Our data highlight the importance of a functioning chaperone-proteasome system for parasite development and differentiation. Thus, a violacein-like small molecule might provide a good scaffold for development of a novel probe for examining the molecular chaperone network and/or antiplasmodial drug design.
Keywords: chaperone inhibitor; chemogenomics; malaria; proteostasis; violacein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
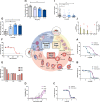

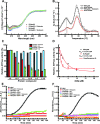
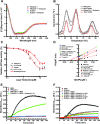
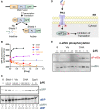
References
-
- World Health Organization . World Malaria Report 2019; Geneva, 2019.
-
- Malaria: Strategy Overview; Bill Melinda Gates Foundation, 2011; April, pp 1–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

