Neoantigen-reactive T cells exhibit effective anti-tumor activity against colorectal cancer
- PMID: 33689574
- PMCID: PMC8920255
- DOI: 10.1080/21645515.2021.1891814
Neoantigen-reactive T cells exhibit effective anti-tumor activity against colorectal cancer
Abstract
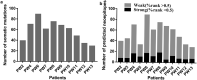
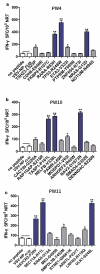
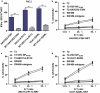
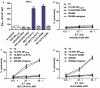
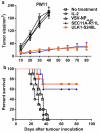
Neoantigens play a crucial role in cancer immunotherapy. However, the effectiveness and safety of neoantigen-based immunotherapies in patients with colorectal cancer (CRC), particularly in the Chinese population, have not been well studied. This study explored the feasibility and effectiveness of neoantigens in the treatment of CRC. Whole-exome sequencing (WES) and transcriptome sequencing were used to identify somatic mutations, RNA expression, and human leukocyte antigen (HLA) alleles. Neoantigen candidates were predicted, and immunogenicity was assessed. The neoantigens TSHZ3-L523P, RARA-R83H, TP53-R248W, EYA2-V333I, and NRAS-G12D from Patient 4 (PW4); TASP1-P161L, RAP1GAP-S215R, MOSPD1-V63I, and NAV2-D1973N from Patient 10 (PW10); and HAVCR2-F39V, SEC11A-R11L, SMPDL3B-T452M, LRFN3-R118Q, and ULK1-S248L from Patient 11 (HLA-A0201+PW11) induced a heightened neoantigen-reactive T cell (NRT) response as compared with the controls in peripheral blood lymphocytes (PBLs) isolated from patients with CRC. In addition, we identified neoantigen-containing peptides SEC11A-R11L and ULK1-S248L from HLA-A0201+PW11, which more effectively elicited specific CTL responses than the corresponding native peptides in PBLs isolated from HLA-A0201+PW11 as well as in HLA-A2.1/Kb transgenic mice. Importantly, adoptive transfer of NRTs induced by vaccination with two mutant peptides could effectively inhibit tumor growth in tumor-bearing mouse models. These data indicate that neoantigen-containing peptides with high immunogenicity represent promising candidates for peptide-mediated personalized therapy.Abbreviations: CRC: colorectal cancer; DCs: dendritic cells; ELISPOT: enzyme-linked immunosorbent spot; E:T: effector:target; HLA: human leukocyte antigen; MHC: major histocompatibility complex; Mut: mutant type; NGS: next-generation sequencing; NRTs: neoantigen-reactive T cells; PBMCs: peripheral blood mononuclear cells; STR: short tandem repeat; PBLs: peripheral blood lymphocytes; PBS: phosphate-buffered saline; PD-1: programmed cell death protein 1; TILs: tumor-infiltrating lymphocytes; RNA-seq: RNA sequencing; Tg: transgenic; TMGs: tandem minigenes; WES: whole-exome sequencing; WT: wild-type.
Keywords: Colorectal cancer; cancer vaccine; immunotherapy; neoantigens; tumor.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Anti-cancer immune effect of human colorectal cancer neoantigen peptide based on MHC class I molecular affinity screening.Front Immunol. 2024 Oct 16;15:1473145. doi: 10.3389/fimmu.2024.1473145. eCollection 2024. Front Immunol. 2024. PMID: 39559350 Free PMC article.
-
Personal Neoantigens From Patients With NSCLC Induce Efficient Antitumor Responses.Front Oncol. 2021 Apr 13;11:628456. doi: 10.3389/fonc.2021.628456. eCollection 2021. Front Oncol. 2021. PMID: 33928024 Free PMC article.
-
Neoantigen-specific immunity in low mutation burden colorectal cancers of the consensus molecular subtype 4.Genome Med. 2019 Dec 30;11(1):87. doi: 10.1186/s13073-019-0697-8. Genome Med. 2019. PMID: 31888734 Free PMC article.
-
Neoantigens in Hematological Malignancies-Ultimate Targets for Immunotherapy?Front Immunol. 2019 Dec 20;10:3004. doi: 10.3389/fimmu.2019.03004. eCollection 2019. Front Immunol. 2019. PMID: 31921218 Free PMC article. Review.
-
Determinants for Neoantigen Identification.Front Immunol. 2019 Jun 24;10:1392. doi: 10.3389/fimmu.2019.01392. eCollection 2019. Front Immunol. 2019. PMID: 31293573 Free PMC article. Review.
Cited by
-
The T cell receptor β chain repertoire of tumor infiltrating lymphocytes improves neoantigen prediction and prioritization.Elife. 2024 Oct 28;13:RP94658. doi: 10.7554/eLife.94658. Elife. 2024. PMID: 39466298 Free PMC article.
-
Circ-MALAT1 accelerates cell proliferation and epithelial mesenchymal transformation of colorectal cancer through regulating miR-506-3p/KAT6B axis.Kaohsiung J Med Sci. 2023 Sep;39(9):862-872. doi: 10.1002/kjm2.12698. Epub 2023 Jun 5. Kaohsiung J Med Sci. 2023. PMID: 37272875 Free PMC article.
-
Correlation analysis of disulfidptosis-related gene signatures with clinical prognosis and immunotherapy response in sarcoma.Sci Rep. 2024 Mar 26;14(1):7158. doi: 10.1038/s41598-024-57594-x. Sci Rep. 2024. PMID: 38531930 Free PMC article.
-
Neoantigen Cancer Vaccines: Generation, Optimization, and Therapeutic Targeting Strategies.Vaccines (Basel). 2022 Jan 26;10(2):196. doi: 10.3390/vaccines10020196. Vaccines (Basel). 2022. PMID: 35214655 Free PMC article. Review.
-
Neoantigens and their clinical applications in human gastrointestinal cancers.World J Surg Oncol. 2022 Sep 29;20(1):321. doi: 10.1186/s12957-022-02776-y. World J Surg Oncol. 2022. PMID: 36171610 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
